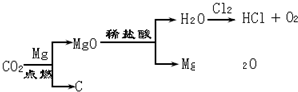
��Ŀ����
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�������������1������200g��������Ϊ8%������������Һ��
�ټ��㣺��Ҫ�������ƹ��������Ϊ______g��ˮ�����Ϊ______mL��ˮ���ܶȽ��ƿ���1g/cm3����
�ڳ���������������ƽƽ�⣬��һ���ձ�����������ƽ��______�̣�������������Ȼ��______�����������Ⱥ�˳��ѡ����ĸ����ֱ����ƽƽ�⣮
A�����������ƹ�������ձ��� B������Ҫ�������롢�ƶ�����
�ò��������ձ�������ֽ�����������Ƶ�ԭ����______��
���ܽ⣺����Ͳ��ȡ�����ˮ������ʢ���������ƹ�����ձ�����裬ʹ���ܽ⣬����ȴ�����£�
�ܰ���õ���Һװ���Լ�ƿ��������Ƥ�������ϱ�ǩ��
��2����ͼ��ʾ���������Ƶ�����������Һ��20gijϡ���ᷢ����Ӧ����Һ�¶ȵı仯�����
�ٸ��������жϣ�����������������Һ������Ϊ______ʱ����Ӧǡ����ȫ���У�
�ڵ�����15g����������Һʱ��������Һ�е�����Ϊ______��д��ѧʽ����
���Լ����ϡ���������ʵ�������������д��������̣���

���𰸡���������1����������������=��Һ����×������������������������Һʱ�����������Ƶ������������ܼ�����=��Һ����-��������������������ˮ����������ʹ��m=��V�����ˮ�������
��ʹ����ƽ����һ�����Ĺ���ҩƷʱ���ȵ�����ƽƽ�⣬�������롢�ƶ����������������������Ȼ�������̵��ձ��ڼ���������������ƽƽ�⣻�����������������տ�����ˮ�������Ҿ��к�ǿ�ĸ�ʴ�ԣ��ڳ�����������ʱ��Ҫ���ڲ��������ڽ��У���ֹ�������Ƹ�ʴ��ƽ���̣�
��2�����������������ᷴӦ�ų�����ʹ��Һ�¶������ߣ�ǡ�÷�Ӧʱ�¶���ߣ�Ȼ����Һ���¶Ƚ��ͣ�
�ڸ����¶ȱ仯���ߣ�����15g����������Һʱ������δ��ȫ��Ӧ����ʱ������ҺΪ��Ӧ�����Ȼ�����δ��ȫ��Ӧ������Ļ����Һ��
���������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������ǡ����ȫ��Ӧʱ�������������Ƶ�����������Ϸ���ʽ���㷴Ӧ�����������ʵ��������ٸ������ʵ������������
����⣺��1����200g��������Ϊ8%������������Һ���������Ƶ�����=200g×8%=16g����������Ҫˮ������=200g-16g=184g������184mL����
�ڸ��ݡ��������롱��ԭ����ʱҩƷ�������ƽ���̣�����һ����ҩƷʱ��Ӧ�ȼ������롢�ƶ�����������Ҫ������������Ȼ�����ҩƷ��ƽ�⣻
�����������ƾ��к�ǿ�ĸ�ʴ�ԣ�Ϊ��ֹ�������Ƹ�ʴ��ƽ�����̣�Ӧ���������Ʒ��벣�������ڽ��г�����
�ʴ�Ϊ��
��16��184��
����BA���������ƾ��и�ʴ�ԣ������������׳��⣩��
��2���ٸ����¶ȱ仯���ߣ����¶ȴﵽ��ߵ�ʱǡ����ȫ��Ӧ����ʱ���μ�����������Һ������Ϊ20g��
�����¶ȱ仯���߿�֪������������������Һ��20gʱǡ����ȫ��Ӧ����ˣ�����15g����������Һʱ����δ��ȫ��Ӧ����ʱ������ҺΪ�Ȼ�����Һ������Ļ����Һ����Һ������ΪHCl��NaCl��
�ʴ�Ϊ����20g����HCl��NaCl��
��20g����������Һ��NaOH������=20g×8%=1.6g
���ϡ������HCl������Ϊx
HCl+NaOH=NaCl+H2
36.5 40
x 1.6g
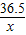 =
=
x=1.46g
ϡ�������������Ϊ�� ×100%=7.3%
×100%=7.3%
�𣺸�ϡ���������ʵ���������Ϊ7.3%��
���������⽫��Һ�����ƺͷ���ʽ����ܺõĽ�ϣ����Ĺؼ����ɷ�Ӧ���¶ȱ仯���ߣ��ҵ����������������Һǡ�÷�Ӧ�ĵ㣬�����н���ܽϺõĿ���ѧ������������������������һ����������Ŀ��
��ʹ����ƽ����һ�����Ĺ���ҩƷʱ���ȵ�����ƽƽ�⣬�������롢�ƶ����������������������Ȼ�������̵��ձ��ڼ���������������ƽƽ�⣻�����������������տ�����ˮ�������Ҿ��к�ǿ�ĸ�ʴ�ԣ��ڳ�����������ʱ��Ҫ���ڲ��������ڽ��У���ֹ�������Ƹ�ʴ��ƽ���̣�
��2�����������������ᷴӦ�ų�����ʹ��Һ�¶������ߣ�ǡ�÷�Ӧʱ�¶���ߣ�Ȼ����Һ���¶Ƚ��ͣ�
�ڸ����¶ȱ仯���ߣ�����15g����������Һʱ������δ��ȫ��Ӧ����ʱ������ҺΪ��Ӧ�����Ȼ�����δ��ȫ��Ӧ������Ļ����Һ��
���������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������ǡ����ȫ��Ӧʱ�������������Ƶ�����������Ϸ���ʽ���㷴Ӧ�����������ʵ��������ٸ������ʵ������������
����⣺��1����200g��������Ϊ8%������������Һ���������Ƶ�����=200g×8%=16g����������Ҫˮ������=200g-16g=184g������184mL����
�ڸ��ݡ��������롱��ԭ����ʱҩƷ�������ƽ���̣�����һ����ҩƷʱ��Ӧ�ȼ������롢�ƶ�����������Ҫ������������Ȼ�����ҩƷ��ƽ�⣻
�����������ƾ��к�ǿ�ĸ�ʴ�ԣ�Ϊ��ֹ�������Ƹ�ʴ��ƽ�����̣�Ӧ���������Ʒ��벣�������ڽ��г�����
�ʴ�Ϊ��
��16��184��
����BA���������ƾ��и�ʴ�ԣ������������׳��⣩��
��2���ٸ����¶ȱ仯���ߣ����¶ȴﵽ��ߵ�ʱǡ����ȫ��Ӧ����ʱ���μ�����������Һ������Ϊ20g��
�����¶ȱ仯���߿�֪������������������Һ��20gʱǡ����ȫ��Ӧ����ˣ�����15g����������Һʱ����δ��ȫ��Ӧ����ʱ������ҺΪ�Ȼ�����Һ������Ļ����Һ����Һ������ΪHCl��NaCl��
�ʴ�Ϊ����20g����HCl��NaCl��
��20g����������Һ��NaOH������=20g×8%=1.6g
���ϡ������HCl������Ϊx
HCl+NaOH=NaCl+H2
36.5 40
x 1.6g
 =
=
x=1.46g
ϡ�������������Ϊ��
 ×100%=7.3%
×100%=7.3%�𣺸�ϡ���������ʵ���������Ϊ7.3%��
���������⽫��Һ�����ƺͷ���ʽ����ܺõĽ�ϣ����Ĺؼ����ɷ�Ӧ���¶ȱ仯���ߣ��ҵ����������������Һǡ�÷�Ӧ�ĵ㣬�����н���ܽϺõĿ���ѧ������������������������һ����������Ŀ��

��ϰ��ϵ�д�
�����Ŀ
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
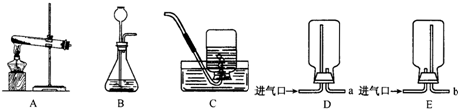
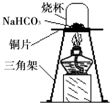
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������