��Ŀ����
����Ŀ��ijС��� H2O2 ��Һ��ȡ��������������̽����
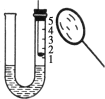
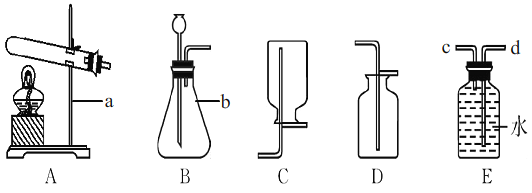
��̽��һ��̽����ͬ������ H2O2�ֽ����ʵ�Ӱ���С�����������ͼ��ʾװ�ý���ʵ�飬ʵ���д�����Ϊ0.4g��H2O2��Һ��Ϊ20mL��Ũ�Ⱦ�Ϊ 10������C�������ӵ��ܺ���Ͳ���������ռ��� 50mL ˮʱ��ij�����ݣ� ���������Ƴ��±���
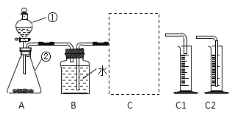
��1����� A װ�������Եķ����ǣ��õ��ɼм�ס A װ���ҲེƤ�ܣ����Ϸ������ӣ� �����м�ˮ���ٵĻ�������__________�������������á�
��2��C ����ѡ��C1��C2װ�ã��Ը�̽��ʵ���������Ӱ��__________��
��3����ʵ�����ݿ�֪����ͬ�����£����д����Ĵ�Ч����ǿ��������Ϊ______��
�������� | ����������ʣ�mL/s�� |
�������� | 3.5 |
����ͭ | 4.5 |
����̿ | 5.8 |
��4����̽��ʵ���У���Ҫ�ⶨ��������__________��
��5������Ͳ���ռ��� 50mL ˮʱ��H2O2�ֽ�������������______50mL������ڡ��� �����ڡ�����С�ڡ�����
��̽������̽�� MnO2 ��������H2O2�ֽ����ʵ�Ӱ����ͼ����ʾװ���в�����������������ѹǿ������������ڵ��������£��������������װ����ѹǿ�����ȡ���Ӧ���Ⱥ��Բ��ƣ�
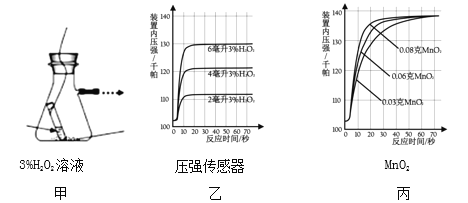
��1��ͼ���ǡ�0.1gMnO2 �벻ͬ����� 3%H2O2 ��Һ��ϡ���ʵ��������ͼ�п��Կ���______________________��
��2�����á�3%H2O2 ��Һ 8 �����벻ͬ������ MnO2 ��ϡ�ʱ���õ���ͼ����ʾ�����ߡ����ߵ�б����ʾ���� MnO2 ���������ӵ� 0.08g ʱ��H2O2 �ķֽ����ʴﵽʵ��Ҫ���ڴ�ʵ�������£���MnO2������Ϊ4g��һҩ�ף�ʱ����ʹ��ͼ____ ��ѡ����ĸ���ձ�����װ��3%H2O2��Һ�� H2O2 �ķֽ�������ӽ�ʵ��Ҫ��
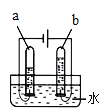
��̽������̽�� H2O2 ��Һ��Ũ�ȶ� H2O2 �ֽ����ʵ�Ӱ����ͼ��ʾ����250mL����ƿ�о�����0.5gMnO2���ں�ѹ©���и�����20mL��ͬŨ�ȵ� H2O2 ��Һ���ֱ����ʵ�顣���¶ȴ��������������Ƴ�ƿ���¶���ʱ��Ĺ�ϵͼ������ͼ 1 ��ʾ���ٽ���װ�õ�����ƿ����ˮԡ���У��óؿ�ʹƿ�ڵ���Һ�¶Ⱥ㶨�� 20������������ʵ������ͬ���ĸ������ظ�����ʵ�飬����ѹ������������ ���Ƴ�ƿ����ѹ��ʱ��Ĺ�ϵͼ������ͼ 2 ��ʾ��


��ͬŨ��˫��ˮ���ֽ�ʱ�¶���ʱ���ϵͼ��ͼ 2-��ͬŨ��˫��ˮ���ֽ�ʱ��ѹ��ʱ���ϵͼ
��1��ʵ���У���ѹ©����������__________��
��2����ͼ 1 ֪����H2O2�ֽ�ʱ��__________��������ų��������ա�����
��3����ͼ2֪����H2O2��ҺŨ��Խ�ߣ�H2O2 �ֽ�����Խ______����족���������� �� 10��H2O2 ��ҺΪ����Լ 25S ������ƿ����ѹ�ɸ������͵�ԭ����_______��
��4��Ӱ�� H2O2 �ֽ����ʵ����أ����˴��������ࡢ������������H2O2 ��Һ��Ũ���⣬��������_______ ��_______�ȡ�
���𰸡�һ��ʱ�������Һ©���е�ˮ�������� �ޣ� ����̿������ͭ������������ �ռ�50mL���������ʱ���� С�ڣ� H2O2��Һ������Խ��������������Խ���� C�� ʹ��ѹ©��������ƿ����ѹ��ȣ�����H2O2��Һ�µΣ� �ų��� �죻 ��Ӧ����������ֹͣ������װ��ɢ�ȣ��¶Ȼ��½���ʹ������ƿ����ѹ��ʼ������С�� ����������С ����������Һ���¶�
��������
[̽��һ] ��1�����ݼ��װ�������Եķ��������
��2������װ�õ����÷��������
��3������ʵ�����ݿ���֪����Ӧ����Խ������Ч��Խ�ý��н����
��4������Ϊ��ɴ�̽������Ҫ�ⶨ���������ռ�50mL���������ʱ����
��5�����ݹ���������Һ�ֽ�ų������������������������������������
[̽����] ��1������ͼ�����ݷ��������
��2������ͼ�и����ı����������
[̽����] ��1������ʵ��������ѹ©����������ʹ��ѹ©��������ƿ����ѹ��ȣ�����H2O2��Һ�µν����
��2����3������ͼ�����ݷ��������
��4������Ӱ�����������Һ�ֽ�����ط��������
[̽��һ] ��1����� A װ�������Եķ����ǣ��õ��ɼм�ס A װ���ҲེƤ�ܣ������Ϸ������ӣ������м�ˮ�������Ļ�������һ��ʱ�������Һ©���е�ˮ�����£���������������
��2��C1 �� C2 װ�ý����ڲⶨBװ�����ų�ˮ�����������C1 �� C2 װ�ö�Bװ�����ų�ˮ�������Ӱ�졣��C ����ѡ�� C1 �� C2 װ�ã��Ը�̽��ʵ�������Ӱ����
��3����ʵ�����ݿ�֪����ͬ�����£����д����Ĵ�Ч����ǿ��������Ϊ������̿������ͭ������������
��4����̽��ʵ���У���Ҫ�ⶨ���������ռ�50mL���������ʱ����
��5������Ͳ���ռ��� 50mL ˮʱ��H2O2�ֽ�������������С��50mL��
[̽����]��1����ͼ�ҿ�֪������3%H2O2��Һ�������������װ����ѹǿ���������ڵ��������£��������������װ����ѹǿ�����ȡ��ʴ�ͼ�п��Կ�����H2O2��Һ������Խ��������������Խ����
��2����ͼʾ��֪3%H2O2��Һ8������0.08��MnO2��ϣ��ֽ��ٶȴﵽʵ��Ҫ����MnO2��������4����Ҫ3%H2O2��ҺΪ![]() ��8mL=400mL����ѡC��
��8mL=400mL����ѡC��
[̽����] ��1��ʵ���У���ѹ©����������ʹ��ѹ©��������ƿ����ѹ��ȣ�����H2O2��Һ�µ���
��2����ͼ 1 ֪��������H2O2��Һ�ķֽ����¶���������H2O2�ֽ�ʱ��ų�������
��3����ͼ2֪����H2O2��ҺŨ��Խ�ߣ�H2O2 �ֽ�����Խ���� ��10��H2O2 ��ҺΪ����Լ 25S������ƿ����ѹ�ɸ������͵�ԭ���ǣ���Ӧ����������ֹͣ������װ��ɢ�ȣ��¶Ȼ��½���ʹ������ƿ����ѹ��ʼ������С��
��4��Ӱ��H2O2�ֽ����ʵ����أ����˴��������ࡢ������������H2O2��Һ��Ũ���⣬�������д���������С������������Һ���¶ȵȡ�

����Ŀ���˽̰��»�ѧ�̲Ĺ��ڡ��ֽ���������������ķ�Ӧ�ж������̵Ĵ����á����Լ���Ѱ���µĴ������о���ʵ�飬�����˻�ѧ̽��С�����Ȥ��
��ʵ��̽����
ʵ�鲽�� | ʵ������ |
�ֱ���ȡ5mL5%����������Һ����A��B��֧�Թ��У���A�Թ��м���ag������( Fe2O3)��ĩ�����ֱ���A��B��֧�Թ��в��������ľ�����۲����� | A�Թ��в������ݣ�������ľ����ȼ��B�Թ������������� |
��A�Թ���û��������ʱ�����¼������������Һ�����Ѵ����ǵ�ľ�������Թܣ���˷������ʵ�飬�۲����� | �Թ��о��������ݣ�������ľ������ȼ�� |
��ʵ����е�ʣ����С�Ĺ��ˣ�����������������ϴ�ӡ�������������ù���������Ϊag�� | |
�����ֱ���ȡ5mL5%����������Һ����C��D��֧�Թ��У���C�Թ��м���ag��������ĩ����D�Թ��м���ag�������̷�ĩ���۲����� |
��ʵ����ۣ�
��1��A�����������______��
��2��ʵ���֤����������(Fe2O3)��_____��________�ڷ�Ӧǰ���û�з����仯����������������ֽ�Ĵ�����
��3��д��������(Fe2O3)����������ֽ����ѧ��������ʽ___________��
��ʵ�����ۣ�
��1�����ʵ�����Ŀ����__________________________��
��2����ʵ����۲쵽D�Թ��в������ݵ����ʸ��죬�ɴ�����Եõ��Ľ�����_______��
��ʵ����չ��
�������ϵ�֪��CuO��CuSO4�����Ρ���������Ҳ����������������Һ�ֽ�Ĵ����������йش�����˵������ȷ����____��
A��MnO2ֻ����Ϊ����������Һ�ֽ�Ĵ���
B��ͬһ����ѧ��Ӧ�����ж��ִ���
C������ֻ�ܼӿ컯ѧ��Ӧ������
D���������������ʲ�������������Ӧ�ķ�Ӧ���������