��Ŀ����
����Ŀ����Һ�ڹ�ũҵ���������ǵ��ճ�������������Ҫ��Ӧ�ã�����������й���Һ�����⡣
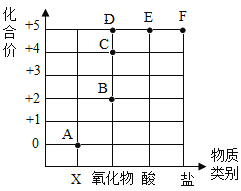
��1�����������ʼ���ˮ�У���ֽ������γ���ɫ��Һ����__�������ţ�
����� ��ʳ�� �۸������ �ܱ�
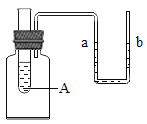
��2���������ʼ���ˮ�г�����ˮ��Ӧ���ܽ���ˮ�С���Щ���̳�����������ת�����������ʼ���A�Թ��У�U����aҺ������ߵ���___�������ţ�
��ŨH2SO4 ��NH4NO3��CaO
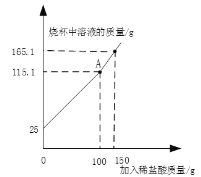
��3�������ڼ䣬���dz���75%�ľƾ�������75%�ľƾ���75����ľƾ����ܶ�0.8g/ml����25�����ˮ���ܶ�1.0g/mL����϶��ɡ���þƾ���Һ��������������Ϊ___��������һλС����
��4��������ˮ����ã�˵��������ˮ�е��ܽ�Ⱥ��¶ȵĹ�ϵ�ǣ�___��
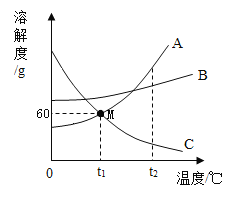
��5��NaCl��Na2CO3�������е�������Ҫ���ʡ��������������ڲ�ͬ�¶�ʱ���ܽ�ȼ��ܽ�����ߡ��ش��������⣺
�¶� | 0�� | 10�� | 20�� | 30�� | 40�� |
NaCl�ܽ��/g | 35 | 35.5 | 36 | 36.5 | 37 |
Na2CO3�ܽ��/g | 6 | 10 | 18 | 36.5 | 50 |

�ٴ���ͼ������NaC1��Na2CO3�ܽ����ȵ��¶���_________��
�ڽ�t2��Cʱ��������NaCl��Na2CO3������Һͬʱ���µ�t1��C�����˵õ�NaCl��������Na2CO3����Y��NaCl��ҺQ��Na2CO3��ҺW�����й��ھ������Һ��˵����ȷ����_____��
A X<Y
B ��Һ��ˮ��Q<W
C ��Һ������������Q=W
���𰸡��� �� 70.6% �¶�Խ�ߣ��ܽ��ԽС 30��C A
��������
��1������۲�����ˮ�����ܺ�ˮ�γɾ�һ���ȶ��Ļ��������ܹ��γ���Һ����ѡ�����
��ʳ��������ˮ���γɾ�һ���ȶ��Ļ���������Һ�����γɵ�����ɫ��Һ����ѡ����ȷ��
�۸����������ˮ���γɾ�һ���ȶ��Ļ���������Һ�����γɵ����Ϻ�ɫ��Һ����ѡ�����
�ܱ�����ˮ���ڻ�����ˮ����ֻ��һ�����ʣ����ܳ�Ϊ��Һ����ѡ�����
��ѡ�ڡ�
��2����Ũ��������ˮ�ų��������ȣ���Һ�¶����ߣ�ƿ��������������������ʹU���е�aҺ�潵�ͣ���ѡ�����
������粒�������ˮʱ������������Һ�¶Ƚ��ͣ�ƿ���������������С��ʹU���е�aҺ�����ߣ���ѡ����ȷ��
������������ˮ�ų��������ȣ���Һ�¶����ߣ�ƿ��������������������ʹU���е�aҺ�潵�ͣ���ѡ�����
��ѡ�ڡ�
��3�� ��
��
��4��������ˮ����ã�˵��������ˮ�е��ܽ�Ⱥ��¶ȵĹ�ϵ���¶�Խ�ߣ��ܽ��ԽС��
��5���ٸ��ݱ�����Կ�����NaC1��Na2CO3�ܽ����ȵ��¶���30����
��A����ͼ�п��Կ���̼���Ƶ��ܽ�����¶�Ӱ����Ȼ��ƴ�ͬʱ���µ�t1����̼���������ľ�����Ȼ��ƶ࣬X��Y����A��ȷ��
B����t2��ʱ�������ı���NaCl��Na2CO3��Һ������t1����̼���������ľ���϶࣬������Һ��������ϵ��NaCl��Һ����>Na2CO3��Һ��������t1��ʱ�������ܽ����ͬ����NaCl��Һ��ˮ������Q��Na2CO3��Һ��ˮ������W����B����
C����t2��ʱ�������ı���NaCl��Na2CO3��Һ������t1�棬����Һ���DZ�����Һ���ܽ����ͬ�����Ա�����Һ��������������ͬ������Ϊ̼������������϶࣬����̼����ʣ�����Һ���٣���Һ����������=��Һ������������������������̼������Һ�������������٣�Q��W����C����
��ѡA��

����Ŀ��KCl��KNO3�ڲ�ͬ�¶��µ��ܽ�����������ʾ������˵������ȷ����
�¶�/�� | 20 | 30 | 60 | |
�ܽ��/g | KCl | 33 | 38 | 45 |
KNO3 | 31 | 64 | 110 |
A. 60��ʱ��100gˮ������ܽ�45 g KCl
B. �����¶ȵ����ߣ�ij����KCl��Һ�л��й�������
C. KNO3���ܽ��ʼ�մ���KCl
D. 60��ʱ��ijKNO3��Һ�����ʵ���������һ��Ϊ ![]()