��Ŀ����
����Ŀ��С��ͬѧ����һƿ��ͯ��Ƭ��Ϊ�ⶨ���ǩ��̼��ƺ����ı�ע�Ƿ���ʵ������������ʵ�飺ȡ��10Ƭ����������С�ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����30g������С�ձ���ʣ�����ʵ�����Ϊ43.4g���ٶ������ɷֲ������ᷴӦ��������㣺

��1��˵������˵�IJ���ָ���Dz����_____������Ԫ��������ԭ���������ӣ���
��2�����ɶ�����̼������_____g��
��3������ÿƬ��Ƭ��̼��Ƶ�����_____��д��������̣�
��4���ø�Ƭ��̼��ƺ�����ע��_____��������ʵ����������ʵ�����ġ�
��5��С��ͬѧ��ȡ10Ƭ��Ƭ����ε���ϡ���ᣬ��������ͼ�л������������������μ�ϡ������������仯��ͼ��_____��
���𰸡�Ԫ�� 6.6 1.5g ��ʵ 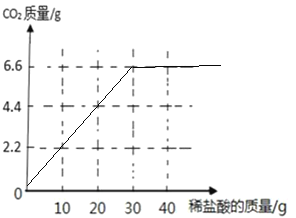
��������
��1����������Ԫ����ɣ�˵������˵�IJ���ָ���Dz����Ԫ�أ�
��2����ÿƬ������Ϊ2g��10Ƭ��Ƭ������Ϊ2g��10=20g�������������غ��֪������̼������=20g+30g-43.4g=6.6g��
��3����10Ƭ��Ƭ��̼��Ƶ�����Ϊxg����
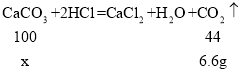
![]()
���x=15g
��ÿƬ��̼��Ƶ�����=![]() ��
��
��4�����Ƭ��Ʒ��ǩͼ�еĹ���б��ÿƬ��̼��Ƶĺ�����1.5g�����ע��ʵ��
��5���������ϼ����֪��10Ƭ��Ƭ��30gϡ���ᷴӦ����6.6g������̼�����������������μ�ϡ������������仯��ͼ��Ϊ�� ��
��

����Ŀ����������ش����⣮
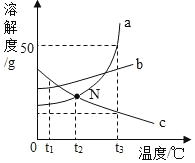
�¶ȣ��棩 | 20 | 40 | 50 | 60 | 80 | |
�ܽ�� ��g/100gˮ�� | NaCl | 36.0 | 36.6 | 37.0 | 37.3 | 38.4 |
NH4Cl | 37.2 | 45.8 | 50.4 | 55.2 | 65.6 | |
KNO3 | 31.6 | 63.9 | 85.5 | 110 | 169 | |
��20��ʱ���ܽ������������
��50��ʱ��100gˮ������ܽ�NaCl g
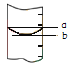
����Ͳ�ľֲ�ʾ���ͼ����ȡˮʱӦ�� ���ߣ�ѡ�a����b�������ж����� ���ߣ�ѡ�a����b������Ӧ�Ķ����ϴ�
��A��80�溬��120gˮ��KNO3��Һ���������²������õ�102gKNO3���壮
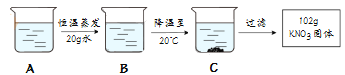
I��A��ҺΪ ��ѡ����͡������͡�����Һ
II�������Ϲ��̵ķ�������ȷ���� ��ѡ���ţ�
a��A��B�Ĺ����У���������û�иı�
b��B���������ܼ���������Ϊ169��100
c����ʼ����KNO3������¶���60����80��֮��
d��A��Һ����������222g��