��Ŀ����
ij��ѧʵ��С��ʵ�����ʱ��������CuSO4��ZnSO4��FeSO4�ķ�Һ���ڷ�Һ���Ϊ�����йؽ������Σ�ͬѧ�����������ʵ�鷽����
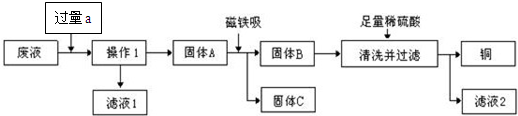
�Իش�
��1������1��
��2����������a���������ԭ����
��3��Ҫ��������ϡ�����Ƿ������ķ�����
��4����ʵ������е�������ʧ���Ժ��ԣ�������������п������

�Իش�
��1������1��
����
����
������A�ijɷ�Cu��Zn��Fe
Cu��Zn��Fe
������C������
����
����Һ1����Һ2��������ͬ����������ZnSO4
ZnSO4
����2����������a���������ԭ����
����Һ�е�Cu2+��Fe2+ȫ���û�����
����Һ�е�Cu2+��Fe2+ȫ���û�����
����3��Ҫ��������ϡ�����Ƿ������ķ�����
ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�����������������
ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�����������������
����4����ʵ������е�������ʧ���Ժ��ԣ�������������п������
��
��
�����������������=����ԭ��Һ������п��������Ҫ����÷�Һ������ͭ����������Ҫ��������D����ͭ��
����D����ͭ��
����������������1����ʵ�����ͼ���жϣ�����1��ɺ�ԭ�����ֳ��˹������Һ�����֣�������һ�ص���жϲ���1Ϊ���˲���������ͭ��п�������ֽ����Ļ���ж�������Һ�м������п�ۺ����ù������ɣ�п������ͭ���ֽ��������У�ֻ�����ܱ��������������ʹ�ô��������������Ͻ�����Ϊ���飬һ��Ϊ������һ��Ϊͭ�ۺ�п�۵Ļ������ݺ����ʵ����жϹ���CΪ���ۣ�����п����CuSO4��ZnSO4��FeSO4�����Һ��Ӧ��������ҺΪZnSO4��Һ��ͭ����п�۵Ļ����������ϡ���ᷴӦ��������ҺΪZnSO4��Һ��
��2�����ݼ��������п�����Һ�е�����ͭ�û��ĸ�����һЩ���н��
��3�����ݲ�����е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п���н��
��4�����ݵõ�������п������Ӧ�ñȷ�Һ�е�����п��������Ϊ��Ӧ����������п�ļ����ʹ����������ˣ�Ҫ��������ͭ��������ͨ�����õ���ͭ��������ɽ��н��
��2�����ݼ��������п�����Һ�е�����ͭ�û��ĸ�����һЩ���н��
��3�����ݲ�����е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п���н��
��4�����ݵõ�������п������Ӧ�ñȷ�Һ�е�����п��������Ϊ��Ӧ����������п�ļ����ʹ����������ˣ�Ҫ��������ͭ��������ͨ�����õ���ͭ��������ɽ��н��
����⣺��1������1�ѻ�������ɹ������Һ�����ԣ�����1Ϊ���˲��������ݽ������п������ͭ������CuSO4��ZnSO4��FeSO4�ķ�Һ���������п�ۣ�����ͭ���û���������˹��˺����ù���A�к�������ͭ��δ��Ӧ��п�����ô����������������ص㣬���жϹ���B��CΪ���ۻ�ͭ����п�۵Ļ����֮һ�����ڹ���B��������ϡ���ᷴӦ��ɵõ�ͭ�ۣ���ȷ������BΪͭ����п�۵Ļ���������CΪ���ۣ����ݽ����Ļ��п�������⣾ͭ����CuSO4��ZnSO4��FeSO4�����Һ�м������п�ۺ���ˣ�������ҺΪZnSO4��Һ����ͭ����п�۵Ļ�����������ϡ�����г�ַ�Ӧ����ˣ�������ҺΪZnSO4��Һ��
�ʴ�Ϊ�����ˣ�Cu��Zn��Fe�����ۣ�ZnSO4��
��2����Ϊֻ�м��������п�Ż����Һ�е�����ͭ�û��ĸ�����һЩ�ڴ˹����з�����п������ͭ����������ͭ�ķ�Ӧ��
�ʴ�Ϊ������Һ�е�Cu2+��Fe2+ȫ���û�������
��3��������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п�������������û������������
�ʴ�Ϊ��ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�������������������
��4����Ϊ��Ӧ����������п�ļ����ʹ��õ�������п��������ˣ��ɹ���֪ͭ����Դֻ������ͭ����������ͭ�����������õõ�ͭ��������
�ʴ�Ϊ����������D����ͭ����
�ʴ�Ϊ�����ˣ�Cu��Zn��Fe�����ۣ�ZnSO4��
��2����Ϊֻ�м��������п�Ż����Һ�е�����ͭ�û��ĸ�����һЩ�ڴ˹����з�����п������ͭ����������ͭ�ķ�Ӧ��
�ʴ�Ϊ������Һ�е�Cu2+��Fe2+ȫ���û�������
��3��������е����Ƿ�������Ҫ������D���Ƿ���δ��Ӧ��п�������������û������������
�ʴ�Ϊ��ȡ����ܵ��������Թ��У���������ϡ���ᣬ�������ݲ�����������������������ݲ�������������������
��4����Ϊ��Ӧ����������п�ļ����ʹ��õ�������п��������ˣ��ɹ���֪ͭ����Դֻ������ͭ����������ͭ�����������õõ�ͭ��������
�ʴ�Ϊ����������D����ͭ����
�����������Ƕ����ʷ���֪ʶ�Ŀ��飬����ʱֻҪץס��Ӧ�����н���������Һ�ķ�Ӧʵ�ʣ��������ص�ʵ����̾���˳�����⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
ʵ��һ��̽��̼��������Һ�������
ȡ̼��������Һ�������μ���ɫ��̪��Һ��죬�ɴ˿�֪̼��������Һ��______�ԣ�
ʵ�����̽��̼�����Ƶ����ȶ���
[��������]̼�������������ֽ⣬����ˮ��������̼�����һ�ֳ����Ĺ������ʣ�
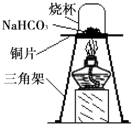
[����ʵ��]Ϊ��֤̼����������ʱ��ֽ⣬��ȤС���ͬѧȡһ��������̼�����Ƶ�ͭƬ�ϼ��ȣ���ͼ��ʾ��
��1������һ��ʱ��۲쵽�ձ��ڱ���______���ɣ�
��2����ּ��Ⱥ��ձ�Ѹ�ٵ�ת���������������ij���ʯ��ˮ�����۲쵽ʯ��ˮ����ǣ�д���÷�Ӧ�Ļ�ѧ����ʽ��______��
��3����ȤС���ͬѧ��Ϊ��ּ��Ⱥ�Ĺ�����������NaOH��Na2CO3��
����ȤС���ͬѧ�����������______��
�������ʵ����鷴Ӧ��Ĺ��������NaOH��Na2CO3���������±���
| ʵ���顡�١��� | Ԥ��ʵ������ | �ᡡ���� |
| ȡ���������������м���______�� | �����ݲ��� | ���������Na2CO3��������NaOH�� |
 ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽����
ij��ѧ��ȤС���ͬѧ���֣����ͷ��ܹ�ʹ������������������Ϊ���ͷ۲�����CO2���£����ͷ۵���Ҫ�ɷ�����̼�����ƣ�NaHCO3�����׳�С�մ����Ƕ�̼�����Ƶ����ʽ�����̽���� ��ѧʵ���г�����������������������������ѧ�����������û��ᣬҪ���ݾ����������Դ������ڷ��ֺͽ�����⣮���磺
��ѧʵ���г�����������������������������ѧ�����������û��ᣬҪ���ݾ����������Դ������ڷ��ֺͽ�����⣮���磺