��Ŀ����
����Ŀ��ͬѧ�ǿ���ͨ�����з�ʽ��ʶ������
����ɽǶȣ�
�ڵ�����������������������̼���������������С�����ѧ������д��
���ǿ�������ɳɷֻ����������������__�������ڴ�����������������_____������ʱ�̶��벻������������Ϊ��_____�� ����������ЧӦ��������_____, д��ʵ���Ҽ��������Ļ�ѧ����ʽ_________
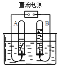
��Ϊ�ⶨ������������������������ͼʵ�顣
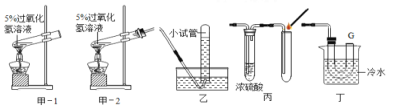
I����ʵ���к�����Ҫ������ԭ����__________��
II������ȼ�յ�������_________����Ӧ�Ļ�ѧ����ʽ_____________��
III����ȴ�����º��ֹˮ�й۲쵽��������_______��
ijͬѧ�ø�װ�����ⶨ�����������������ʵ��ʱ�������������趼��ȷ����ֹˮ��û�н��������ⶨ���������������� ____���� ƫ���ƫС��Ӱ�죩
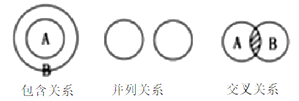
���۽Ƕ� ��![]()
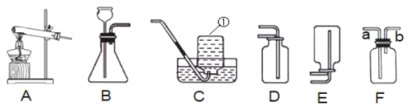
���á���ѧ���š���ͼʾ����ա�
ͼ ʾ |
| ______ |
|
��ѧ���� | ___ | N2 | _____ |
��ͬ��ͬѹ�£����������ȵ��ڷ��Ӹ����ȡ������Կ����������ɷ֣���ͼ�ɱ�ʾ������ģ�͵���________����ѡ���
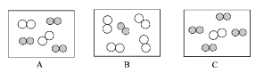
���仯�Ƕȣ�
��һ��������ѹ�£������в�����ֵķе����£�
��� | ���� | ���� | ������̼ |
�е㣨�棩 | -195.8 | -183.0 | -78.4 |
ȼ�ŵ�ľ������ʢ��Һ̬������-200�棩�ĸ�ƿ�ڣ��۲쵽��������____��
�����������������_____��
A��ʳƷ��װ�ڳ�N2�Է�������ΪN2�Ļ�ѧ���ʱȽ��ȶ���
B����ҵ�Ϸ���Һ̬������ȡ�����ǻ�ѧ�仯
C���ӱ������ó���ˮ��ƿ�������Һ�飬˵����������ˮ����
D�����ó���ʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ��֤���������ж�����̼
���𰸡�SO2 He O2 CO2 CO2+Ca(OH)2![]() CaCO3
CaCO3 ![]() +H2O ʹ������ַ�Ӧ�� �������̣��ų����� 4P �� 5O2
+H2O ʹ������ַ�Ӧ�� �������̣��ų����� 4P �� 5O2 ![]() 2P2O5 �ձ��е�ˮ��������ƿ��Լ1/5��� ƫ�� 2N
2P2O5 �ձ��е�ˮ��������ƿ��Լ1/5��� ƫ�� 2N ![]() 2NO2 C ȼ�ŵ�ľ��Ϩ�� B
2NO2 C ȼ�ŵ�ľ��Ϩ�� B
��������
��ɽǶ����ٸ��ݿ����ijɷ����ʼ����ɷֵ�����������������
�ڸ��ݲⶨ�����������������ʵ���ԭ���������ۡ������ķ�Ӧ��ע������������
�۽Ƕ����ٸ������Ĺ��ɡ���ѧʽ����������ش�
�ڸ��ݿ�������ɷ����ж���
�仯�Ƕ����ٸ��ݷ���Һ̬������������ԭ������������֧��ȼ�ս����
�ڸ��ݿ����ijɷֵ����ʽ��з������
��ɽǶ������ڵ�����������������������̼���������������У����������ǿ����ijɷ֣����������ڿ������γ����ꡣ���Ի���������������Ƕ�����������ѧʽ��SO2���������ܶȽ�С�һ�ѧ�ȶ��������ڴ��������������ѧʽ��He�������ܹ�������������ʱ�̶��벻������������Ϊ����������ѧʽ��O2�� ����������ЧӦ�������Ƕ�����̼��������̼��ʹ����ʯ��ˮ����ǣ����������������̼��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ����ѧ����ʽ��CO2+Ca(OH)2![]() CaCO3
CaCO3 ![]() +H2O��
+H2O��
��I����ʵ���ԭ�����������ڿ�����ȼ�գ�����������������ѹǿ��С��ʹˮ�������������ݽ���������ˮ�������ȷ���������������������ʵ���к�����Ҫ������ԭ����ʹ������ַ�Ӧ�꣬ʵ������ȷ��
II������ȼ�յ������Ǵ������̣��ų����������ڿ�����ȼ���������������ף���Ӧ�Ļ�ѧ����ʽ4P ��5O2 ![]() 2P2O5��
2P2O5��
III����ȴ�����º��ֹˮ�й۲쵽���������ձ��е�ˮ��������ƿ��Լ1/5�����ijͬѧ�ø�װ�����ⶨ�����������������ʵ��ʱ�������������趼��ȷ����ֹˮ��û�н���ʵ������еIJ��ֿ����ݳ������ⶨ����������������ƫ��
�۽Ƕ����� ��![]() ����ʾ��ԭ�ӣ��� ��
����ʾ��ԭ�ӣ��� ��![]() ����ʾ2����ԭ�ӣ�����Ϊ��2N��N2������������ԭ�ӹ��ɵģ�ͼʾΪ����
����ʾ2����ԭ�ӣ�����Ϊ��2N��N2������������ԭ�ӹ��ɵģ�ͼʾΪ����![]() ������
������![]() ����ʾ1����������2����ԭ�Ӻ�1����ԭ������ʾ����������������
����ʾ1����������2����ԭ�Ӻ�1����ԭ������ʾ����������������![]() �� ��ʾ���������������ӣ�����Ϊ2NO2��
�� ��ʾ���������������ӣ�����Ϊ2NO2��
�����ڿ����е������������Լռ������4/5������Լռ���������1/5���������������������4��1������ͬ��ͬѹ�£����������ȵ��ڷ��Ӹ������������Կ����������ɷ֣���ͼ�ɱ�ʾ������ģ�͵���C��
�仯�Ƕ����ٷ���Һ̬������������������Һ����Һ���ķе㲻ͬ��Һ���ķе�ͣ���������������������֧��ȼ�գ����Խ�ȼ�ŵ�ľ������ʢ��Һ̬�������ձ��ڣ��۲쵽��������ȼ�ŵ�ľ��Ϩ��
��A��N2�Ļ�ѧ���ʱȽ��ȶ�������ʳƷ��װ�ڳ�N2�Է�������A��ȷ��B����ҵ�Ϸ���Һ̬���������������͵����ķе㲻ͬ����ȡ������û�����������ɣ����������仯����B����C������ӱ������ó�������Χ�а������ǿ����е�ˮ����Һ���ɵ�Сˮ�Σ���C��ȷ��D����ʢʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ������Ϊʯ��ˮ���տ����еĶ�����̼���ɲ����Ե�̼��Ƶ�Ե�ʣ���D��ȷ����ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�����ᷢչ��Ƽ����������˾��ס��ϳɰ����յ���Ҫ�������£�
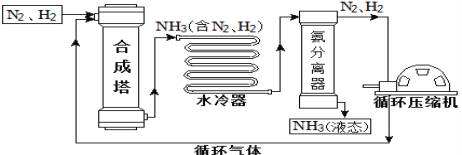
��1���ϳ����еķ�Ӧ�����ڸ��¡���ѹ�����������½��У��÷�Ӧ�Ļ�ѧ����ʽ�ǣ�________
��2�����������п��ظ�ʹ�õ�������_______________���ѧʽ����
��3�����ݱ��е����ݻش����⡣
���� | H2 | N2 | O2 | NH3 |
�е�/�棨1.01��105 Pa�� | �C252 | �C195.8 | �C183 | �C33.35 |
��1.01��105 Paʱ��Ҫ������NH3��N2��H2���뿪���������˵��¶�Ӧ�ÿ�����_________�档
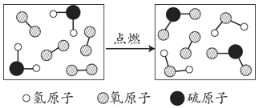
��4����ͼ�Ǻϳ����з�����Ӧ��������ʾ��ͼ��
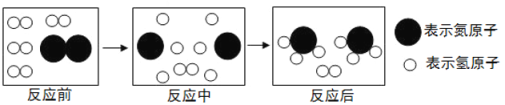
��ͼ��֪���ַ�Ӧ��N2��H2�ķ��Ӹ�����Ϊ____________���÷�Ӧ�е���С������_________����д��ѧ���ţ���