��Ŀ����
(3��) �����죬Сǿ������Ҫ�����������Сǿȥ�̵����һ�����Сǿ��ϸ���˰�װ˵��(����ͼ)�����������ʣ�������Ʒ��̼���Ƶ����������Ƿ����أ�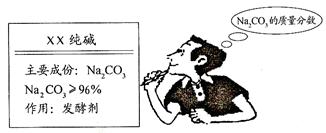
�ص�ѧУ����ȡ���Ӽ��������һС��������Ʒ��С��һ�����ʵ�飬С����Сǿ�ֱ�������������ֲ�ͬ��ʵ�鷽����
| | ʵ�鷽�� |
| Сǿ | ȷ��ȡ5.5 g������Ʒ�����ձ��У���ȡ��50�ˡ�14.6%��ϡ������μ��룬���պò��ٲ�������ֹͣ�μ�ϡ���ᣬ��ʣ��ϡ����25�ˡ� |
| С�� | ȷ��ȡ5.5 g������Ʒ������ƿ�У�����һ������������������ϡ���ᣬ������������������ͨ������������������Һ�У�����ͼ��ʾ�� ��������պ�������������Һ����������2.0�ˡ�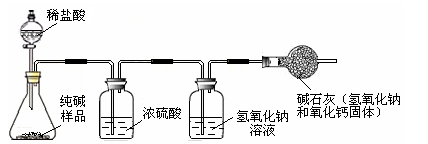 |
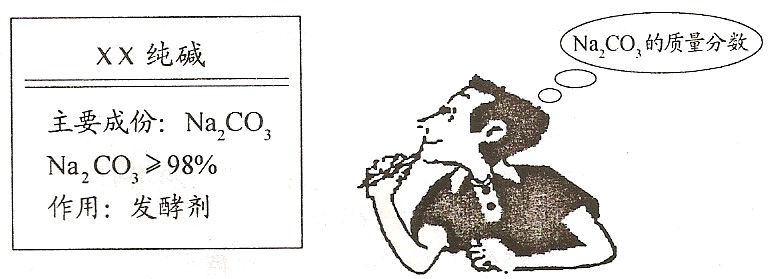
��2��С����õĴ�����Ʒ��̼���Ƶ����������Ƕ��٣���д��������̣�
��3����λͬѧ��������ʦ��æ����ʦѡȡ����һ�ֲⶨ�����������ⶨ�������96.3%��������ʵ����̷���һ����λͬѧ˭�IJⶨ�����ȷ��Ϊʲô��
(1)96.4% ��2��87.6%
��3��С����ʵ���в��������岻��ȫ��������������Һ���գ�������������һ���֣�ʹ�òⶨ�����ȷ��
����������������ݻ�ѧ����ʽ���㼴�ɡ�
��1���⣺�贿����Ʒ��̼���Ƶ�����Ϊx�� +2HCl="2NaCl+" H2O+ CO2��
+2HCl="2NaCl+" H2O+ CO2��
106 73
x 25g��14.6%
106:73=x��25g��14.6%
x=5.3g
������Ʒ��̼���Ƶ�����������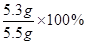 =96.4%��
=96.4%��
�𣺴�����Ʒ��̼���Ƶ�����������96.4%��
��2���⣺�贿����Ʒ��̼���Ƶ�����Ϊy�� +2HCl="2NaCl+" H2O+ CO2��
+2HCl="2NaCl+" H2O+ CO2��
106 44
y 2.0g
106:44=y��2.0g
y=4.8g
������Ʒ��̼���Ƶ�����������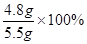 =87.6%��
=87.6%��
�𣺴�����Ʒ��̼���Ƶ�����������87.6%��
��3����ΪС����ʵ���в��������岻��ȫ��������������Һ���գ�������������һ���֣�ʹ�òⶨ�����ȷ��
���㣺���ݻ�ѧ����ʽ���㣻ʵ�������̽����
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
Ҫ��ʵ��Ľ��ȷ����ƿ�ѧ������ʵ�����Ҫ��

(3��) �����죬Сǿ������Ҫ�����������Сǿȥ�̵����һ�����Сǿ��ϸ���˰�װ˵��(����ͼ)�����������ʣ�������Ʒ��̼���Ƶ����������Ƿ����أ�
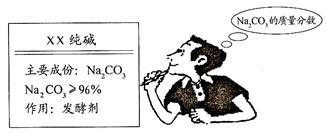
�ص�ѧУ����ȡ���Ӽ��������һС��������Ʒ��С��һ�����ʵ�飬С����Сǿ�ֱ�������������ֲ�ͬ��ʵ�鷽����
|
|
ʵ�鷽�� |
|
Сǿ |
ȷ��ȡ5.5 g������Ʒ�����ձ��У���ȡ��50�ˡ�14.6%��ϡ������μ��룬���պò��ٲ�������ֹͣ�μ�ϡ���ᣬ��ʣ��ϡ����25�ˡ� |
|
�� |
ȷ��ȡ5.5 g������Ʒ������ƿ�У�����һ������������������ϡ���ᣬ������������������ͨ������������������Һ�У�����ͼ��ʾ�� ��������պ�������������Һ����������2.0�ˡ�
|
��1��Сǿ��õĴ�����Ʒ��̼���Ƶ����������Ƕ��٣���д��������̣�
��2��С����õĴ�����Ʒ��̼���Ƶ����������Ƕ��٣���д��������̣�
��3����λͬѧ��������ʦ��æ����ʦѡȡ����һ�ֲⶨ�����������ⶨ�������96.3%��������ʵ����̷���һ����λͬѧ˭�IJⶨ�����ȷ��Ϊʲô��