��Ŀ����
(08ɽ��̩��)(4��)�����죬Сǿ������Ҫ�����������Сǿȡ�̵����һ�����Сǿ��ϸ���˰�װ˵��(����ͼ)�����������ʣ�
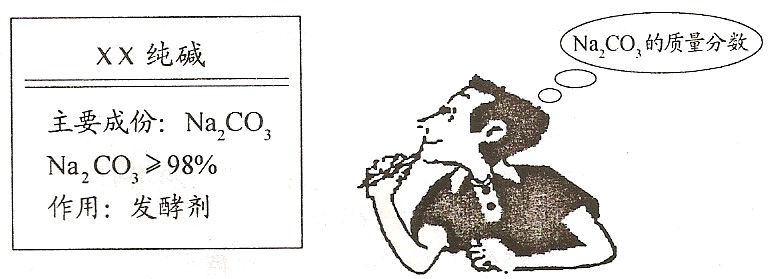
�ص�ѧУ����ȡ���Ӽ��������һС��������Ʒ����ʵ�飺ȷ��ȡ5.5 g��Ʒ�����ձ��У��ڵμ��������պ���ȫ��Ӧ(����CO2����ˮ)������ȥϡ����25 g������Һ����Ϊ28.3 g(��������ˮ���������Ӧ)����
(1)����CO2�������� ��
(2)ͨ�������жϴ�����Ʒ��̼���Ƶ����������Ƿ����װ˵�������(��������ȷ��0.1%)
(08ɽ��̩��)(4��)(1)2.2 g(1��)
(2)�⣺��5.5 g��Ʒ�к���̼���Ƶ�����Ϊx
Na2CO3��2HCl=2NaCl��CO2����H2O
106 44
x 2.2 g������������������������������1��
106�U44=x�U2.2 g
���x=5.3 g������������������������������������������������1��
����Ʒ��̼���Ƶ���������Ϊ![]() ��96.4%
��96.4%
��Ϊ96.4%��98%���������װ˵����������������������������1��

��ϰ��ϵ�д�
�����Ŀ