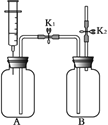
ΧβΡΩΡΎ»ί
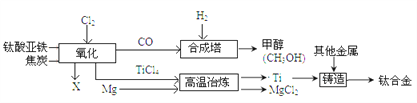
ΓΨΧβΡΩΓΩ‘γ‘Ύ¥Κ«ο’ΫΙζ ±ΤΎΘ§Έ“ΙζΨΆΩΣ Φ…ζ≤ζΚΆ Ι”ΟΧζΤςΘ§ΙΛ“Β…œΝΕΧζΒΡ‘≠άμ «άϊ”Ο“Μ―θΜ·ΧΦΚΆ―θΜ·ΧζΖ¥”ΠΘ§Ρ≥Μ·―ß–Υ»Λ–ΓΉιάϊ”Ο»γΆΦΉΑ÷ΟΫχ–– Β―ιΧΫΨΩΘ§«κΑ¥“Σ«σΧνΩ’ΘΚ
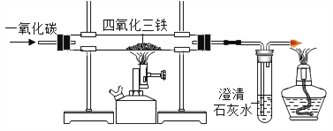
(1)–¥≥ωCOΜΙ‘≠―θΥΡ―θΜ·»ΐΧζΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ_________ΓΘ
(2)ΡήΥΒΟς“Μ―θΜ·ΧΦΜΙ‘≠ΥΡ―θΜ·»ΐΧζ τ”ΎΜ·―ß±δΜ·ΒΡœ÷œσ «________ΓΘ
(3)”“±ΏΒΦΤχΙήΩΎΖ≈÷ΟΒψ»ΦΒΡΨΤΨΪΒΤΒΡΡΩΒΡ «______________ΓΘ
(4)»τΑ―ΥΡ―θΜ·»ΐΧζΜΜ≥…“ΜΕ®ΝΩΒΡ¥≈ΧζΩσ―υΤΖWΩΥ(ΗΟΖ¥”ΠΆξ»ΪΫχ––)Θ§≤Δœκ≤βΕ®ΗΟ¥≈ΧζΩσ―υΤΖ÷–ΥΡ―θΜ·»ΐΧζΒΡ÷ ΝΩΖ÷ ΐΘ§–η“Σ≤βΝΩΒΡ ΐΨί «_____________ΓΘ
ΓΨ¥πΑΗΓΩ 4CO+Fe3O4ΗΏΈ¬3Fe+4CO2 ≥Έ«ε ·Μ“Υ°±δΜκΉ« ≥ΐ»ΞΈ≤Τχ÷–CO ≥δΖ÷Ζ¥”ΠΚσ,≤ΘΝßΙή÷– Θ”ύΙΧΧεΒΡ÷ ΝΩ
ΓΨΫβΈωΓΩΘ®1Θ©“Μ―θΜ·ΧΦ”κΥΡ―θΜ·»ΐΧζΖ¥”Π…ζ≥…ΧζΚΆΕΰ―θΜ·ΧΦΘ§Ι ΖΫ≥Χ ΫΈΣ4CO+Fe3O4ΗΏΈ¬3Fe+4CO2
Θ®2Θ©ΡήΥΒΟς“Μ―θΜ·ΧΦΜΙ‘≠ΥΡ―θΜ·»ΐΧζΒΡœ÷œσ «≥Έ«ε ·Μ“Υ°±δΜκΉ«
Θ®3Θ©ΗΟ Β―ι÷–”–“Μ―θΜ·ΧΦ≤ΈΦ”Ζ¥”ΠΘ§“Μ―θΜ·ΧΦ «”–ΕΨΒΡΤχΧεΘ§ΈΣΝΥΖά÷Ι“Μ―θΜ·ΧΦΈέ»ΨΩ’ΤχΘ§”ΟΨΤΨΪΒΤΒψ»Φ“Μ―θΜ·ΧΦΘΜ
Θ®4Θ©≤βΕ®ΗΟ¥≈ΧζΩσ―υΤΖ÷–ΥΡ―θΜ·»ΐΧζΒΡ÷ ΝΩΖ÷ ΐΘ§–η“Σ≤βΝΩΒΡ ΐΨί «―υΤΖΒΡΉή÷ ΝΩΚΆΖ¥”ΠΚσ≤ΘΝßΙή÷– Θ”ύΙΧΧεΒΡ÷ ΝΩΓΘ

ΓΨΧβΡΩΓΩΒγ≥Ί «»’≥Θ±Ί±Η”ΟΤΖ÷°“ΜΘ§ΒΪΥϋ“≤ «ΜΖΨ≥Έέ»ΨΒΡ“ΜΗω÷Ί“Σά¥‘¥ΓΘœ¬Οφ «Ρ≥–Υ»Λ–ΓΉιάϊ”ΟΖœΨ…–ΩΟΧΗ…Βγ≥ΊΉςΈΣ‘≠ΝœΘ§≤ΔΫχ––œύΙΊΧΫΨΩΒΡΙΐ≥ΧΓΘ
Θ®÷Σ Ε¥Δ±ΗΘ©
(1)–ΩΟΧΒγ≥ΊΒΡΙΙ‘λΚΆΉι≥…Θ®ΦϊΆΦΘ©ΓΘ

(2)ΫαΨßΥ°ΚœΈοΒΡΧΊ β–‘÷ ΘΚ‘Ύ÷π≤Ϋ…ΐΗΏΈ¬Ε»ΧθΦΰœ¬Θ§ΫαΨßΥ°ΚœΈοΡή ß»Ξ≤ΩΖ÷Μρ’Ώ»Ϊ≤ΩΫαΨßΥ°Θ§»γάΕ…ΪΒΡΒ®Ζ·ΨßΧεΘ®CuSO4 5H2OΘ© ή»» ±Ω… ß»ΞΫαΨßΥ°±δΈΣΑΉ…ΪΒΡΈόΥ°ΝρΥαΆ≠ΖέΡ©Θ®CuSO4Θ©ΓΘ
I.÷Τ±Ηπ©Ζ·ΨßΧεΘ®ZnSO4xH2OΘ©
–ΓΉιΆ§―ß≤ΈΙέΝΥΡ≥ΜΊ ’ΖœΨ…–ΩΟΧΒγ≥ΊΒΡΙΛ≥ßΘ§ΤδΜΊ ’ΙΛ“’Νς≥Χ»γΆΦΘΚ

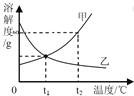
(1)Νς≥ΧΆΦ÷– ‘ΦΝaΒΡΜ·―ß Ϋ «________ΘΜΒΟΒΫΒΡ¬Υ“Κ1≈©“Β…œΩ…”ΟΉς_______________ΓΘ
(2)ΫΪ¬Υ‘ϋB‘ΎΩ’Τχ÷–≥δΖ÷ΉΤ…’Ω…Χα¥Ω÷ΤΒΟΒΡΙΧΧε «_________Θ§ΗΟΖΫΖ®Χα¥ΩΒΡ‘≠άμ «Θ®”ΟΜ·―ßΖΫ≥Χ ΫΜΊ¥πΘ©______________________ΓΘ
Θ®3Θ©≤βΕ®–ΩΤΛΘ®Ά≠–ΨΘ©÷––ΩΒΡ÷ ΝΩΖ÷ ΐΘΚ≥Τ»Γ≤ΜΆ§÷ ΝΩ–ΩΤΛΘ®Ά≠–ΨΘ©”Ύ…’±≠÷–Θ§≤ΔΦ”»κΒ»≈®Ε»ΒΡœΓΝρΥαΘ§ ΐΨί»γœ¬±μΥυ ΨΘΚ
–ΩΤΛΘ®Ά≠–ΨΘ©ΒΡ÷ ΝΩ | 20g | 15g |
œΓΝρΥαΒΡ÷ ΝΩ | 100g | 120 g |
…ζ≥…ΤχΧεΒΡ÷ ΝΩ | 0.4 g | 0.4 g |
«σΘΚ–ΩΤΛΘ®Ά≠–ΨΘ©÷––ΩΒΡ÷ ΝΩΖ÷ ΐΓΘΘ®–¥≥ωΦΤΥψΙΐ≥ΧΘ§¥πΑΗ±ΘΝτ“ΜΈΜ–Γ ΐΘ©_______
(4)¬Υ“Κ2÷–»ή÷ ÷ς“Σ «ΝρΥα–ΩΘ§Τδ”–ΙΊ»ήΫβΕ»ΚΆΈ¬Ε»ΙΊœΒ»γœ¬±μΘ§
Έ¬Ε»/Γφ | 0 | 20 | 40 | 60 | 80 | 100 | |
»ήΫβΕ»/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
ΫΪ¬Υ“Κ2’τΖΔ≈®ΥθΓΔ_____________ΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΘ§Ω…ΒΟΒΫπ©Ζ·ΨßΧεΘ®ZnSO4xH2O)ΓΘ
–ΓΉιΆ§―ßΫΪ≤ΩΖ÷π©Ζ·ΨßΧεΘ®ZnSO4xH2OΘ©¥χΜΊ Β―ι “Θ§”Ο»γΆΦΉΑ÷Ο≤βΕ®ΨßΧε÷–ΫαΨßΥ°ΒΡΚ§ΝΩΘ®ΆΦ÷–ΑκΆΗΡΛΩ…»ΟΤχΧεΆ®Ιΐ”÷Ω…Ζά÷ΙΙΧΧεΖέΡ©Ϋχ»κΒΦΙήΘ©ΓΘ≤βΕ®ΖΫΖ®ΘΚ≥Τ»Γ28.7gΨßΧε÷Ο”ΎCΉΑ÷ΟΒΡ”≤÷ ≤ΘΝßΙή÷–Θ§Φ”»»÷ΝΆξ»Ϊ ß»ΞΫαΨßΥ°:

Θ®ZnSO4xH2O ==== ZnSO4 + xH2OΘ©Θ§ά以÷Ν≥ΘΈ¬ΚσΘ§≥ΤΝΩ≤ΘΝßΙή÷–ΙΧΧεΤδ÷ ΝΩΈΣ16.1gΓΘ
Θ®5Θ© A÷–ΒΡΜ·―ßΖΫ≥Χ Ϋ «______________Θ§B÷–ΒΡ ‘ΦΝΩ…¥”œ¬Ν–Έο÷ ÷–―Γ»ΓΘ§ΡψΒΡ―Γ‘ώ «________ΓΘ
A.≈®ΝρΥα B.œθΥα“χ»ή“Κ C.±ΞΚΆΧΦΥαΡΤ»ή“Κ D.≥Έ«ε ·Μ“Υ°
Θ®6Θ© Β―ιΙΐ≥Χ÷–»τ≤ΜΆ®»κΩ’Τχ≤βΒΟΒΡΫαΙϊΫΪ____________
Θ®ΧνΓΑΤΪ¥σΓ±ΓΔ ΓΑΤΪ–ΓΓ±ΜρΓΑΈό”ΑœλΓ±Θ©ΓΘΗυΨί Β―ιΫαΙϊΘ§ΦΤΥψπ©Ζ·ΨßΧε÷–ΫαΨßΥ°ΒΡx÷ΒΈΣ_____ΓΘ
Θ®7Θ©ΫΪ…œ ωπ©Ζ·ΨßΧεΦ”»»Μα÷πΫΞ ß»Ξ≤ΩΖ÷ΫαΨßΥ°Θ§Φ”»»Ιΐ≥Χ÷–”–ΙΊ≤–ΝτΙΧΧε÷ ΝΩ»γ”“ΆΦΘ§–¥≥ωC-D
ΕΈΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ____________________ΓΘ

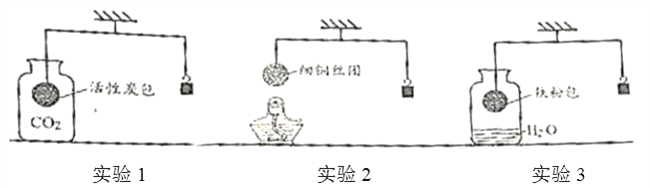
ΓΨΧβΡΩΓΩ Β―ι “”–“ΜΤΩ≥ΛΤΎ±©¬Ε‘ΎΩ’Τχ÷–ΒΡ«β―θΜ·ΡΤΙΧΧε―υΤΖΘ§Ιέ≤λΖΔœ÷Θ§―υΤΖ±μΟφ”–ΑΉ…ΪΖέΡ©ΓΘΡ≥–Υ»Λ–ΓΉιΒΡΆ§―ßΕ‘ΗΟ―υΤΖΒΡ≥…Ζ÷ΦΑΚ§ΝΩΫχ––ΝΥΧΫΨΩΓΘ
Θ®Χα≥ωΈ Χβ1Θ©ΗΟ―υΤΖ÷–Κ§”–ΡΡ–©Έο÷ ΘΩ
Θ®Χα≥ω≤¬œκΘ©≤¬œκΔώΘΚ»Ϊ≤Ω «NaOHΘΜ
≤¬œκΔρΘΚ“―Άξ»Ϊ±δ÷ Θ§ΗΟ―υΤΖ÷–÷ΜΚ§Na2CO3ΘΜ
≤¬œκΔσΘΚ≤ΩΖ÷±δ÷ Θ§ΗΟ―υΤΖ÷–Κ§”–NaOHΚΆNa2CO3ΓΘ
Θ® Β―ιΧΫΨΩ1Θ©ΈΣ»ΖΕ®ΗΟ―υΤΖ≥…Ζ÷Θ§–ΓΟς…ηΦΤΝΥ»γœ¬ Β―ιΖΫΑΗΘ§«κΡψ“ΜΤπΆξ≥…œ¬Ν– Β―ι±®ΗφΓΘ
Β―ι≤ΌΉς | Β―ιœ÷œσ | Β―ιΫα¬έ |
ΔΌ»Γ…ΌΝΩ―υΤΖ»ή”ΎΥ°Θ§Φ”»κ ΉψΝΩΒΡ________ΘΜ | ΑΉ…Ϊ≥ΝΒμ≤ζ…ζ | ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ________ |
ΔΎΫΪ…œ ωΖ¥”ΠΚσΒΡΜλΚœ“ΚΙΐ¬ΥΘ§ »Γ¬Υ“ΚΦ”»κ________ΘΜ | ________ | ÷ΛΟς≤¬œκΔσ≥…ΝΔ |
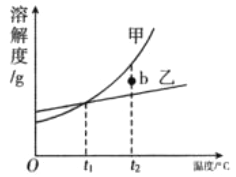
Θ®Χα≥ωΈ Χβ2Θ©‘θ―υΧα¥ΩΗΟ―υΤΖΒΟΒΫ¥ΩΨΜΒΡ«β―θΜ·ΡΤΙΧΧεΘΩ
Θ® Β―ιΧΫΨΩ2Θ©ΈΣΒΟΒΫ¥ΩΨΜΒΡ«β―θΜ·ΡΤΙΧΧεΘ§…ηΦΤΒΡ Β―ιΙΐ≥Χ»γœ¬ΆΦΓΘ«κΜΊ¥πœ¬Ν–Έ Χβ

Δ≈≥ΛΤΎ±©¬Ε‘ΎΩ’Τχ÷–ΒΡ«β―θΜ·ΡΤΙΧΧε―υΤΖΦΪ“Ή±δ÷ Θ§‘≠“ρ «________Θ®”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΘ©ΓΘ≤ΌΉςBΒΡΟϊ≥ΤΈΣ________Θ§Υυ–ηΒΡ≤ΘΝß“«Τς”–________ΓΔ…’±≠ΚΆ≤ΘΝßΑτΓΘ
ΔΤΗΟ Β―ι÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________ΓΘ
Δ«Ήν÷’ΥυΒΟ«β―θΜ·ΡΤΙΧΧε÷ ΝΩ________Θ®―ΓΧνΓΑ<Γ±ΓΔΓΑ=Γ±ΜρΓΑ>Γ±Θ©±δ÷ Κσ―υΤΖ÷–«β―θΜ·ΡΤΒΡ÷ ΝΩΓΘ
Θ® Β―ιΧΫΨΩ3Θ©ΈΣΝΥ≤βΕ®NaOHΒΡ¥ΩΕ»Θ§Ρ≥Ά§―ß…ηΦΤ»γΆΦΉΑ÷ΟΓΘ
“―÷ΣΘΚCO2‘Ύ±ΞΚΆΧΦΥα«βΡΤ»ή“Κ÷–ΦΗΚθ≤Μ»ήΫβΓΘ

Δ»B÷–Φ·ΤχΤΩ ΔΖ≈ΒΡ±ΞΚΆΧΦΥα«βΡΤ»ή“Κ≤ΜΡή”ΟΥ°¥ζΧφΘ§Τδάμ”… «________ΓΘ
Δ…»Γ10g―υΤΖΫχ–– Β―ιΘ§ΗυΨί Β―ι ΐΨίΘ§Ά®ΙΐΦΤΥψΩ…÷Σ≤ζ…ζΝΥCO20.44gΘ§«σ‘≠―υΤΖ÷–NaOHΒΡ÷ ΝΩΖ÷ ΐΘ§–¥≥ωΦΤΥψΙΐ≥Χ_____________ΓΘ
ΓΨΧβΡΩΓΩάϊ”Οœ¬ΆΦΉΑ÷ΟΫχ–– Β―ιΓΘ Β―ι«ΑK1ΓΔK2Ψυ“―ΙΊ±’ΓΘ
Β―ιΉΑ÷Ο | Β―ι1 | Β―ι2 |
| Δώ. A÷– Δ”–Υ°Θ§“ΚΟφΫΰΟΜœ¬ΕΥΒΦΙήΩΎΘ§B÷– Δ”–Κ§Ζ”ΧΣΒΡNaOH»ή“Κ Δρ.ΫΪΉΔ…δΤς÷–ΒΡ≈®ΝρΥαΉΔ»κA÷–Θ§≤Δ±Θ≥÷ΉΔ…δΤςΜν»ϊ≤ΜΕ·Θ§≥δΖ÷Ϋ”¥ΞΚσΘ§¥ρΩΣK1ΚΆK2 | Δώ. A÷–≥δ¬ζCO2Θ§B÷– Δ”–“ΜΕ®ΝΩΒΡΥ° Δρ. ΫΪΉΔ…δΤς÷–ΒΡNaOH»ή“Κ(ΉψΝΩ)ΉΔ»κA÷–Θ§≥δΖ÷Ζ¥”ΠΚσΘ§¥ρΩΣK1ΚΆK2 |
(1)Φλ≤ιAΉΑ÷ΟΤχΟή–‘ΘΚœρœ¬ΆΤΉΔ…δΤςΒΡΜν»ϊΘ§Υ… ÷ΚσΜν»ϊΜ÷Η¥÷Ν‘≠ΈΜΘ§ΗΟœ÷œσΥΒΟς________ΓΘ
(2) Β―ι1Ιέ≤λΒΫA÷–“ΚΧεΝς»κB÷–Θ§B÷–»ή“Κ”…Κλ…Ϊ±δ≥…Έό…ΪΘ§≤ζ…ζΗΟœ÷œσΒΡ‘≠“ρ «_________ΓΘ
(3) Β―ι2≤ΜΡή÷ΛΟςΕΰ―θΜ·ΧΦ”κ«β―θΜ·ΡΤΖΔ…ζΖ¥”ΠΘ§Τδάμ”… «_________________ΓΘ