��Ŀ����
ij��ͬѧ�����ȡ������̼��ʵ��Ի��յ�������Ȼ��ƻ��Һ���������������ʣ���������ʵ�飺ȡһ�����û��Һ���ձ��У��μ�40g������������Ϊ10.6%��̼������Һ�Ƶ��ձ�����Һ������Ϊ68.5g������̼������Һ�����������ɳ���������ϵ��ͼ��ʾ��
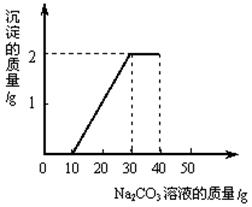
��1��һ��ʼû�г������ɵ�ԭ����̼�����������ᷴӦ���������ᷴӦ��̼������Һ������Ϊ g��
��2������ȡ�ܺ���Һ���Ȼ��Ƶ�������
��3����ǡ����ȫ��Ӧʱ��Һ�����ʵ�����������

��1��һ��ʼû�г������ɵ�ԭ����̼�����������ᷴӦ���������ᷴӦ��̼������Һ������Ϊ g��
��2������ȡ�ܺ���Һ���Ȼ��Ƶ�������
��3����ǡ����ȫ��Ӧʱ��Һ�����ʵ�����������
��1��10g
��2�����Ȼ��Ƶ�����ΪX
Na2CO3+ CaCl2="Ca" CO3��+ 2NaCl
111 100
X 2g
111/100=X/2g,���X=2.22g.
��3������̼���ƺ����ᷴӦ���Ǻ��Ȼ��Ʒ�Ӧ�������ɵ��Ȼ��ƣ����Ը��ݹ���Ӧ��40��̼������Һֱ���������Ȼ��Ƶ����������Ȼ��Ƶ�����ΪY��
Na2CO3-------2NaCl
106 117
40g*10.6% Y
106/117=40g*10.6%/Y
���Y=4.68�ˣ�������Һ����Ϊ68.5g-2=66.5g
��ǡ����ȫ��Ӧʱ��Һ�����ʵ���������4.68/66.5*100%=7%
д��̼�������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ1�֣��ٳ�������Ҳ���֣�����ʽ�淶�����衢�𣬲����g������ʽ��1��
��2�����Ȼ��Ƶ�����ΪX
Na2CO3+ CaCl2="Ca" CO3��+ 2NaCl
111 100
X 2g
111/100=X/2g,���X=2.22g.
��3������̼���ƺ����ᷴӦ���Ǻ��Ȼ��Ʒ�Ӧ�������ɵ��Ȼ��ƣ����Ը��ݹ���Ӧ��40��̼������Һֱ���������Ȼ��Ƶ����������Ȼ��Ƶ�����ΪY��
Na2CO3-------2NaCl
106 117
40g*10.6% Y
106/117=40g*10.6%/Y
���Y=4.68�ˣ�������Һ����Ϊ68.5g-2=66.5g
��ǡ����ȫ��Ӧʱ��Һ�����ʵ���������4.68/66.5*100%=7%
д��̼�������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ1�֣��ٳ�������Ҳ���֣�����ʽ�淶�����衢�𣬲����g������ʽ��1��
��1����ͼ����Կ������ᷴӦ��̼������Һ������Ϊ10g
��2����3���������ɳ���2�ˣ���������Ȼ��Ƶ���������Һ�����ʵ��������������岽����𰸡�
��2����3���������ɳ���2�ˣ���������Ȼ��Ƶ���������Һ�����ʵ��������������岽����𰸡�

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ
