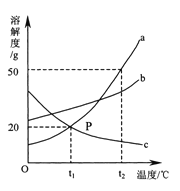
��Ŀ����
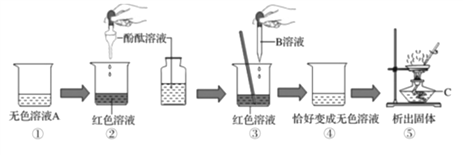
����Ŀ����ͬѧ������ͼװ�öԶ�����̼�����ʽ�����֤��
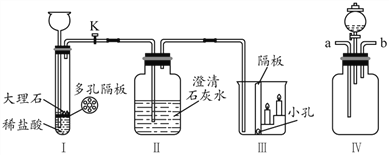
(1)ͼ���д���ʯ����Ҫ�ɷ���___________________ (д��ѧʽ)��
(2)��K����Ӧһ��ʱ���װ�â��з�����Ӧ�Ļ�ѧ����ʽ��_________________ ��
�۲쵽װ�â���___________(�ϲ���²�)������Ϩ��)��
(3)��ͬѧ���â�ͼ��ʾװ����֤������̼��ˮ�ķ�Ӧ��Ϊ˵��ʹʯ���ɫ��������̼�������ˮ�������̼��Ӧ�ò�ȡ��ʵ�����˳����___________________(����������ظ�ѡ��)��
�� ��b��ͨ����
�� �ӷ�Һ©���еμ�����ˮ
�� ��a��ͨ������̼
�� ����ʯ����ҺȾ����ɫ�ĸ���ֽ��������ƿ�У�������ľ��
���𰸡� CaCO3 CO2 + Ca(OH)2 �� CaCO3 ��+ H2O �²� �ܢۢ٢ڢ�
��������������Ҫ���������̼�����ʺ�ʵ����ơ�
(1)ͼ���д���ʯ����Ҫ�ɷ�̼��ƣ���ѧʽ��CaCO3��
(2)��K����Ӧһ��ʱ���װ�â��з����ķ�Ӧ���������ƺͶ�����̼��Ӧ����̼��ƺ�ˮ����ѧ����ʽ��CO2 + Ca(OH)2 ��CaCO3 ��+ H2O��������̼���ܶȱȿ����۲쵽װ�â����²�������Ϩ��
(3)��֤ʹʯ���ɫ��������̼������Ƕ�����̼�����Խ�ʯ����ҺȾ����ɫ�ĸ���ֽ��������ƿ�У���a��ͨ�������̼����ʱ��ʯ����ҺȾ����ɫ�ĸ���ֽ������ɫ��Ȼ���ٴ�b��ͨ�뵪�������ƿ�еĶ�����̼�ų������Ŵӷ�Һ©���еμ�����ˮ��ֽ���Բ���ɫ������ٴ�a��ͨ�������̼����ʱ��ʯ����ҺȾ����ɫ�ĸ���ֽ����졣��ȡ��ʵ�����˳���Ǣܢۢ٢ڢ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�