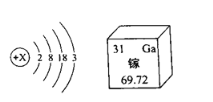
��Ŀ����
����Ŀ��ij�����ĩ��Mg��MgO��Mg (OH)2�е�һ�ֻ�����ɡ�ij��ѧС��Ϊ��̽���ù����ĩ�ijɷ֣������ͼ��ʾ��װ��(�̶�װ��ʡ��)��
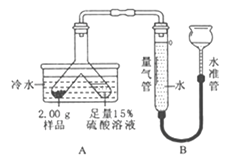
��ʵ����̣�
�����������װ�������ԣ�װ��ҩƷ������������
����������бY�ιܣ������ݲ���������Ʒ��ַ�Ӧ��������������Ϊ55.6mL�����ݱ�ʵ�������������ܶ�ת��Ϊ����ԼΪ0.005�ˡ�
������������װ�ã���Y�ι��ڲ���Һ�м�������������Һ�����������ɳ����������ˡ�ϴ�Ӻ���Ƶó�������Ϊ2.90g��
���ش����⣩
(1)����װ�������Եļ��鷽����:��ˮ������һ�ξ��룬ʹˮ�ܺ��������е�ˮ�γ�һ����Һ�����һ��ʱ���Һ���________(�����ı����������ֲ�����)����˵��װ�ò�©����
(2)��ʹ��Ʒ�������ַ�Ӧ����ȷ������_________(����)��
A. ��Ʒת�Ƶ�������Һ�� B. ������Һת�Ƶ���Ʒ��
���ж���Ʒ��ȫ��Ӧ��������___________________��
(3)����ʵ����
��ͨ���������ƶϣ���Ʒ��һ�����е�������______________��ͨ�����������㣬��Ʒ��þԪ�ص�������Ϊ______________��
�����ۣ�����Ʒ�����ʵijɷ���____________________��
��ʵ�鷴˼��
(4)����˵����ȷ����_______
A.ˮ������ˮ�������Ƿ�ֹ��Ӧ���ȵ���ʵ������ȷ
B.ʵ��ǰ��ͨ����Ʒ��������������Һ��������ȷ����Ʒ��ȫ��Ӧ
C.�����ƶ�ˮ�ܣ�ʹˮ����Һ�����������Һ��һ�����ٶ����������������ñ�ʵ�������������ܶȺ����ת������������
���𰸡� ���ֲ��� B Y�ι��еĹ���ȫ����ʧ þ 1.20g þ������þ��������þ[��Mg��MgO��Mg (OH)2] ABC
��������������Ҫ������ʵ��̽�����ʵ���ɳɷ��Լ����������װ�õ������ԣ���������Ļ�ѧ���ʣ������غ㶨�ɵ����ڽ���ʱ�����ȷ�������������и����ʵ����ʣ�Ȼ���������������ʵ����������жϣ����ȷ����������ɳɷ֡�
(1)����װ�������Եļ��鷽��������ˮ������һ�ξ��룬ʹˮ�ܺ��������е�ˮ�γ�һ����Һ�����һ��ʱ���Һ���ֲ��䣬˵��װ��������û���ݳ�����˵��װ�ò�©����
��2����Ϊ�˷�ֹ����ҩƷճ���������ϣ��ɽ�Һ̬��������Һת�Ƶ���Ʒ�У���ѡB����þ������þ��������þ������ϡ���ᷴӦ�����ɿ����Ե�����þ����������ҩƷ��ȫ��ʧ�ˣ�֤����Ʒ����ȫ��Ӧ�������ж���Ʒ��ȫ��Ӧ��������Y�ι��еĹ���ȫ����ʧ��
��3����þ��ϡ���ᷴӦ��������þ������������þ��������þ��ϡ���ᷴӦ��û���������ɣ��ɲ����ʵ�������֪����Ʒ��һ������þ�� þ������þ��������þ������ϡ���ᷴӦ�����ɿ����Ե�����þ������þ���������Ʒ�Ӧ����������þ�������ƣ����������غ㶨�ɿ�֪��������þ������þԪ�ص�������Ϊ��Ʒ��þԪ�ص���������2.90g��![]() ��100%=1.20g��
��100%=1.20g��
����������0.005g������Ҫþ������Ϊx
Mg+H2SO4=MgSO4+H2��
24 2
x 0.005g
![]() x=0.06g
x=0.06g
��ʣ�����ȫ��Ϊ����þ����þԪ�ص�����Ϊ����2.00g-0.06g����![]() ��100%=1.164g�٣�1.20g-0.06��=1.14g�����Թ�����Ʒ�г���þ������þ����������þ�����Թ�����Ʒ����þ������þ��������þ[��Mg��MgO��Mg (OH)2]����������ɵģ�
��100%=1.164g�٣�1.20g-0.06��=1.14g�����Թ�����Ʒ�г���þ������þ����������þ�����Թ�����Ʒ����þ������þ��������þ[��Mg��MgO��Mg (OH)2]����������ɵģ�
��4��A��þ�����ᷴӦ�Ƿ��ȷ�Ӧ��������¶Ȼ�Ӱ����������ˮ������ˮ�������Ƿ�ֹ��Ӧ���ȵ���ʵ������ȷ����ȷ��B�����ݻ�ѧ����ʽMg+H2SO4=MgSO4+H2����MgO+H2SO4=MgSO4+H2O��Mg(OH)2+H2SO4=MgSO4+2H2O��֪��ÿ24��������þ����98�����������ᣬÿ40����������þ����98���������ᣬÿ58������������þ����98�����������ᣬʵ��ǰ��ͨ����Ʒ������������ȫ��Ϊþ������������Һ��������ȷ����Ʒ��ȫ��Ӧ����ȷ��C�������ƶ�ˮ�ܣ�ʹˮ����Һ�����������Һ��һ�����ٶ����������������������������ǵ�ʱ����ѹ�µ�����������������ñ�ʵ�������������ܶȺ����ת��������������ȷ����ѡABC��

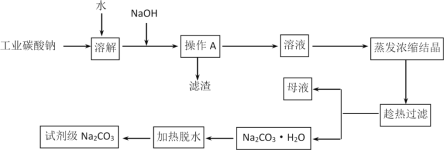
����Ŀ��̼��������ֽ����������֯���Ƹ����ҵ����Ҫԭ�ϡ���ҵ̼���ƣ�����Լ98%���к���Ca2+��Mg2+��Fe3+��Cl-��SO42-�����ʣ��ᴿ�����������£�
̼���Ƶı�����Һ�ڲ�ͬ�¶����������������±���ʾ��
�¶ȣ��棩 | ��32 | 32��36 | ��36 |
������Ļ�ѧʽ | Na2CO3��10H2O | Na2CO3��7H2O | Na2CO3��H2O |
�ش��������⣺
��1������AΪ���ˣ�����IJ����������ձ�����������_____________����������Ҫ�ɷ�ΪFe(OH)3��Mg(OH)2��___________��
��2�������ȹ��ˡ���ԭ����____________________��
��3����ĸҺ���г��˺��� Na+��CO32-��Cl-��OH�������⣬������________�����ӣ�д��һ�����ɣ���
��4��д��Na2CO3��H2Oת��ΪNa2CO3�Ļ�ѧ����ʽ_______________________________________��