��Ŀ����
��2007?��Ǩ���±�ΪijƷ������Ƭ��ǩ�е�һ���֣�
��1������Ƭ����Ϊ�����ṩ������Ӫ������
��2������Ƭ�к���
��3�����彡���벻����Ԫ�أ������йظ�Ԫ�ص������У�����ȷ����
�������и�Ԫ�شִ����ڹ�����������
������������ȱ�ƻ�����Ͳ��ͷ�������
��������ȱ�ƻᷢ���������ɣ�������
�ܳ����˱�������������Ҫ�������ĸ�
��4����Ƭ����Ч�ɷ���̼��ƣ���Ȼ̼���������ˮ�������ú��ת��Ϊ�����Ը��α��������գ���ԭ����
��5����������ʳ��ٴ��עڶ��͢���ܼ�������ˢ����ϣ��������ṩ�ḻ�����ʵ���
| Ӫ���ɷ� ��ÿ100g���У� | ������ | ���� | ��֬ | �� | �� | �� | п | ά����C |
| 7.4g | 7.8g | 7.9g | 206mg | 19.2mg | 37.8mg | 10.1mg | 18mg |
���ࡢ�����ʡ���֬
���ࡢ�����ʡ���֬
����2������Ƭ�к���
2
2
�������������Ԫ�أ���3�����彡���벻����Ԫ�أ������йظ�Ԫ�ص������У�����ȷ����
��
��
������ţ����������и�Ԫ�شִ����ڹ�����������
������������ȱ�ƻ�����Ͳ��ͷ�������
��������ȱ�ƻᷢ���������ɣ�������
�ܳ����˱�������������Ҫ�������ĸ�
��4����Ƭ����Ч�ɷ���̼��ƣ���Ȼ̼���������ˮ�������ú��ת��Ϊ�����Ը��α��������գ���ԭ����
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
���û�ѧ����ʽ��ʾ����Ϊ����߲���Ч�������ø�Ƭʱ������Ϸ���ά����D���ݴ˿���֪ά����D�Ĺ������ٽ�����ԸƵ�����
�ٽ�����ԸƵ�����
����5����������ʳ��ٴ��עڶ��͢���ܼ�������ˢ����ϣ��������ṩ�ḻ�����ʵ���
�ۢ�
�ۢ�
����������1���������ࡢ�����ʡ���֬���������ܻش�
��2�����ݱ�����Ϣ��������Ԫ�ص�����ش�
��3�����ݸ��������ڵĴ��ں��������ܻش�
��4������θ��ijɷ֣��Լ�̼��ƺ�����Ļ�ѧ���ʣ�ά����D�Ĺ��ܻش�
��5�����ݵ����ʵ���Ҫʳ����Դ���Լ����ٴ��עڶ��͢���ܼ�������ˢ���������������ҪӪ���ػش�
��2�����ݱ�����Ϣ��������Ԫ�ص�����ش�
��3�����ݸ��������ڵĴ��ں��������ܻش�
��4������θ��ijɷ֣��Լ�̼��ƺ�����Ļ�ѧ���ʣ�ά����D�Ĺ��ܻش�
��5�����ݵ����ʵ���Ҫʳ����Դ���Լ����ٴ��עڶ��͢���ܼ�������ˢ���������������ҪӪ���ػش�
����⣺��1�����ࡢ�����ʡ���֬�������ڶ��������ֽ⣬ͬʱ�ͷ��������������Ҫ��
��2�������ڵĺ�������0.01%������Ԫ�أ���ǩ�е�����п����������������Ԫ�أ��ƺ����dz���Ԫ�أ�
��3���������и�Ԫ�شִ����ڹ����������У������˹Ǽ�Ӳ��֧�ܣ�������ȷ��
�ڸ��ǹ��ɹǸ�����ݵ���ҪԪ�أ�����������ȱ�ƻ�����Ͳ��ͷ���������������ȷ��
�۸��ǹ��ɹǸ�����ݵ���ҪԪ�أ�������ȱ�ƻᷢ���������ɣ������ۣ�������ȷ��
�������������´������������ڣ��ȳ�������Ҫ�������ĸƣ����Դ���
��4��θ�����Ҫ�ɷ������ᣬ̼��ƺ����ᷴӦ�����ɿ��ܵ��Ȼ��ơ�ˮ��������̼������ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O�����ø�Ƭʱ������Ϸ���ά����D����߲���Ч����˵��ά����D�ܴٽ�����ԸƵ����գ�
��5���ٴ�������Ҫ�������࣬�ʴ���
�ڶ���������֬���ʴ���
���㡢�ܼ�������Ҫ���е����ʣ�����ȷ��
����ˡ�����������Ҫ����ά���أ��ʴ���
�ʴ�Ϊ����1�����ࡢ�����ʡ���֬��
��2��2��
��3���ܣ�
��4��CaCO3+2HCl=CaCl2+CO2��+H2O���ٽ�����ԸƵ����գ�
��5���ۢܣ�
��2�������ڵĺ�������0.01%������Ԫ�أ���ǩ�е�����п����������������Ԫ�أ��ƺ����dz���Ԫ�أ�
��3���������и�Ԫ�شִ����ڹ����������У������˹Ǽ�Ӳ��֧�ܣ�������ȷ��
�ڸ��ǹ��ɹǸ�����ݵ���ҪԪ�أ�����������ȱ�ƻ�����Ͳ��ͷ���������������ȷ��
�۸��ǹ��ɹǸ�����ݵ���ҪԪ�أ�������ȱ�ƻᷢ���������ɣ������ۣ�������ȷ��
�������������´������������ڣ��ȳ�������Ҫ�������ĸƣ����Դ���
��4��θ�����Ҫ�ɷ������ᣬ̼��ƺ����ᷴӦ�����ɿ��ܵ��Ȼ��ơ�ˮ��������̼������ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O�����ø�Ƭʱ������Ϸ���ά����D����߲���Ч����˵��ά����D�ܴٽ�����ԸƵ����գ�
��5���ٴ�������Ҫ�������࣬�ʴ���
�ڶ���������֬���ʴ���
���㡢�ܼ�������Ҫ���е����ʣ�����ȷ��
����ˡ�����������Ҫ����ά���أ��ʴ���
�ʴ�Ϊ����1�����ࡢ�����ʡ���֬��
��2��2��
��3���ܣ�
��4��CaCO3+2HCl=CaCl2+CO2��+H2O���ٽ�����ԸƵ����գ�
��5���ۢܣ�
���������Ե�Ӫ�����Գ��������������ձ����ʳ�ʻ�ѧ�϶�����Ӫ���صĿ���Ҳ�����ȵ�֮һ���ر�������Ӫ���غ�����Ӫ��Ԫ�ذ��������ࡢ�������ܡ�ʳ����Դ��ȱ��֢������ʱ��ע������ȣ�

��ϰ��ϵ�д�
�����Ŀ
��2007?��Ǩ���±�ΪijƷ������Ƭ��ǩ�е�һ���֣�
��1������Ƭ����Ϊ�����ṩ������Ӫ�����У�
��2������Ƭ�к����������������Ԫ�أ�
��3�����彡���벻����Ԫ�أ������йظ�Ԫ�ص������У�����ȷ���ǣ�����ţ���
�������и�Ԫ�شִ����ڹ�����������
������������ȱ�ƻ�����Ͳ��ͷ�������
��������ȱ�ƻᷢ���������ɣ�������
�ܳ����˱�������������Ҫ�������ĸ�
��4����Ƭ����Ч�ɷ���̼��ƣ���Ȼ̼���������ˮ�������ú��ת��Ϊ�����Ը��α��������գ���ԭ���ǣ��û�ѧ����ʽ��ʾ����Ϊ����߲���Ч�������ø�Ƭʱ������Ϸ���ά����D���ݴ˿���֪ά����D�Ĺ����ǣ�
��5����������ʳ��ٴ��עڶ��͢���ܼ�������ˢ����ϣ��������ṩ�ḻ�����ʵ��У�
| Ӫ���ɷ� ��ÿ100g���У� | ������ | ���� | ��֬ | �� | �� | �� | п | ά����C |
| 7.4g | 7.8g | 7.9g | 206mg | 19.2mg | 37.8mg | 10.1mg | 18mg |
��2������Ƭ�к����������������Ԫ�أ�
��3�����彡���벻����Ԫ�أ������йظ�Ԫ�ص������У�����ȷ���ǣ�����ţ���
�������и�Ԫ�شִ����ڹ�����������
������������ȱ�ƻ�����Ͳ��ͷ�������
��������ȱ�ƻᷢ���������ɣ�������
�ܳ����˱�������������Ҫ�������ĸ�
��4����Ƭ����Ч�ɷ���̼��ƣ���Ȼ̼���������ˮ�������ú��ת��Ϊ�����Ը��α��������գ���ԭ���ǣ��û�ѧ����ʽ��ʾ����Ϊ����߲���Ч�������ø�Ƭʱ������Ϸ���ά����D���ݴ˿���֪ά����D�Ĺ����ǣ�
��5����������ʳ��ٴ��עڶ��͢���ܼ�������ˢ����ϣ��������ṩ�ḻ�����ʵ��У�
��2007?��Ǩ�����и�ѡ���У���������ʵ���Ǻϵ��ǣ� ��
| ѡ�� | ��ʵ | ���� |
| �� | ���죬ԶԶ���ŵ�������ζ | �����ڲ�ͣ���˶� |
| �� | ��ѹ�����£�����Һ���������С | ��ѹ�����£����ӵ��������С |
| �� | ˮ��ͨ�������£��ɷֽ�Ϊ���������� | ��ѧ��Ӧ�з����ǿɷֵ� |
| �� | ���ᡢϡ���ᶼ��ʹ��ɫʯ����Һ��� | ���ᡢϡ�����ж����д�����H+ |
��2007?��Ǩ�����и�ѡ���У���������ʵ���Ǻϵ��ǣ� ��
| ѡ�� | ��ʵ | ���� |
| �� | ���죬ԶԶ���ŵ�������ζ | �����ڲ�ͣ���˶� |
| �� | ��ѹ�����£�����Һ���������С | ��ѹ�����£����ӵ��������С |
| �� | ˮ��ͨ�������£��ɷֽ�Ϊ���������� | ��ѧ��Ӧ�з����ǿɷֵ� |
| �� | ���ᡢϡ���ᶼ��ʹ��ɫʯ����Һ��� | ���ᡢϡ�����ж����д�����H+ |
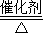 4X+6H2O����X�Ļ�ѧʽΪ��
4X+6H2O����X�Ļ�ѧʽΪ��