��Ŀ����
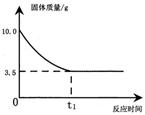
��6�֣����ܶ�Ϊ1.14g/cm3��H2SO4��Һ75ml�����ձ�����������ƽ�������ϣ�����ƽ���̼�����ʹ��ƽƽ�⡣Ȼ�����ձ��м�пƬ���ɣ���Ӧֹͣ��ȡ��ʣ��пƬ������ƽ��������11.0g���룬��ƽ�ִﵽƽ�⡣����Ƶ�����ʣ��пƬ������Ϊ1.65g��
��1�������ձ��е�������Һ����Ϊ g����Ӧ�����Һ������ g
��2����������ձ���пƬ�������� g
��3����Ӧ���������Һ���ʵ����������Ƕ��٣�
��1�������ձ��е�������Һ����Ϊ g����Ӧ�����Һ������ g
��2����������ձ���пƬ�������� g
��3����Ӧ���������Һ���ʵ����������Ƕ��٣�
��1�� 85.5g�� 11.0g ��2�� 13g ��3��29��1%
��1��75mL�ܶ�Ϊ1.14g/cm3��H2SO4��Һ����Ϊ��75��1.14�T85.5g����Ӧ��ɺ�ȡ��пƬ������ƽ����������11g���룬����Һ����11g��
��2����μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2�� ��Ӧ����Һ��������
65 2 65-2=63
x 11g
65/ x=63/11
x=11.35g
����������ձ���пƬ������=11.35g+1.65g=13g
����������ձ���пƬ��������13g��
��3�������⣬������ȫ��Ӧ������������п������Ϊy
Zn+H2SO4�TZnSO4+H2��
65 161
11.35g y
65/11.35="161/" y
y�T28.11g
��Ӧ����Һ������11g���ʷ�Ӧ����Һ����Ϊ��85.5g+11g=96.5g
�ʷ�Ӧ���������Һ���ʵ����������ǣ�28.11��96.5��100%�T29.1%
�𣺷�Ӧ���������Һ���ʵ�����������29.1%
��2����μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2�� ��Ӧ����Һ��������
65 2 65-2=63
x 11g
65/ x=63/11
x=11.35g
����������ձ���пƬ������=11.35g+1.65g=13g
����������ձ���пƬ��������13g��
��3�������⣬������ȫ��Ӧ������������п������Ϊy
Zn+H2SO4�TZnSO4+H2��
65 161
11.35g y
65/11.35="161/" y
y�T28.11g
��Ӧ����Һ������11g���ʷ�Ӧ����Һ����Ϊ��85.5g+11g=96.5g
�ʷ�Ӧ���������Һ���ʵ����������ǣ�28.11��96.5��100%�T29.1%
�𣺷�Ӧ���������Һ���ʵ�����������29.1%

��ϰ��ϵ�д�
�����Ŀ