��Ŀ����
����Ŀ��ѧϰ�����ڿ���������������С����С���ͭ�ڿ�������������������Ũ�����Ȥ�����ǽ���������̽�����̣�
���������ϣ�ͭ������ɫ�ģ��׳�ͭ�̣���Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu��OH��2CO3],ͭ�̼����ֽܷ�����һ�ֳ����������
���²⣩С��²�ͭ����������е����������йأ���CO2 ��O2 ��N2 ��H2O
С����Ϊͭ�����뵪��һ���أ�������_____��
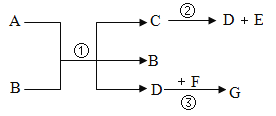
��ʵ��̽����С���������ͼ��ʾ��ʵ�飬�����۲쵽��A�Թ���ͭƬ��ˮ�渽������ɫ���ʣ�B��C��D�Թ���ͭƬ���������������������ʵ����̲��ش��������⣺
��1����������ʵ��ó���ͭ��������������������_____��
��2������ʵ��_____������ű�ʾ��˵��ͭ����������һ���йء�
��ʵ�鷴˼��ͨ��ѧϰ��������Ϊ����ͭ�ڿ����и����������_____��
��̽����չ�����ʵ��̽��ͭ�����ȷֽ����������ʲô��
��3������CO2���ɣ���������CO2�Ļ�ѧ����ʽΪ_____��
��4������������������ͭ���ɹ۲쵽��������_____��
��5����д��ͭ�����ȷֽ�Ļ�ѧ����ʽ_____��
���𰸡�ͭ���в����е�Ԫ�� ˮ��������������̼ AB �� Ca��OH��2+CO2=CaCO3��+H2O ��ɫ������ɫ Cu2��OH��2CO3![]() 2CuO+ H2O+CO2��
2CuO+ H2O+CO2��
��������
[�²�]
ͭ�����뵪��һ���أ�������ͭ���в����е�Ԫ�ء�
���ͭ���в����е�Ԫ�ء�
[ʵ��̽��]
��1����������ʵ��ó���ͭ��������������������ˮ��������������̼��
���ˮ��������������̼��
��2������ʵ��AB˵��ͭ����������һ���йأ�������ΪA�к���������ͭ�ܹ����⣬B�в�������������������.���AB��
[ʵ�鷴˼]
����ͭ�ڿ����и��������������������Ϊ�����ж�����̼�����ϵ͡�
�������
[̽����չ]
��3�����������̼�����ó���ʯ��ˮ��������Ϊ������̼��ʹ�����ʯ��ˮ����ǣ�����Ϊ������̼��ʯ��ˮ�е��������Ʒ�Ӧ������̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��
���Ca��OH��2+CO2=CaCO3��+H2O��
��4������������������ͭ���ɹ۲쵽����������ɫ������ɫ��
�����ɫ������ɫ��
��5��ͭ�����ȷֽ���������ͭ��ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��Cu2��OH��2CO3![]() 2CuO+ H2O+CO2����
2CuO+ H2O+CO2����

����Ŀ�����ꡰ����������ȼ�ա���ʵ���ij��ȤС����һЩ�ɻ�����⣬���ǽ���������̽��:
������һ����ȼ��ʱ���������ĺ�ɫ�����л���û����?
����������1���������������ױ�����Ϊ�������������Ȼ����������������Ҫ������������������������;��������Ϊ����ɫ���壬���ܱ��������������������������ܱ���������;�����ܺ�ϡ���ᷴӦ��������������������Ҳ�ܺ�ϡ���ᷴӦ���������������
��ʵ��̽��������˿ȼ�պ�õ��ĺ�ɫ������ȴ��Ȼ�����飬___________________��˵�����������ĺ�ɫ�����л�������д����˿��������ȼ�յķ��ű���ʽ:_________________��
�����������ȼ�յIJ���Ϊʲô������������?
����������2�����������������������ķֽ��¶ȼ��±�;�����������·ֽ��������������
���������ۣ���������̽���Լ�ʵ��������ϱ������ݷ���������֪����������ȼ��ʱ�����ĸ�����___________֮�䣬���¶ȷ�Χ�������ѷֽ⡣
���������� | ������ | �� | |
�ֽ��¶�/�� | 1538 | 1400 | / |
�۵�/�� | / | / | 1535 |
����չ���죩
��1�����������·ֽ������������������һ�����壬д���÷�Ӧ�ķ��ű���ʽ:______________;
��2����ЩʳƷ��װ���ڷ���������ɫ����������������ĩ����ɫ______________����˵����װ��©�����ۻ�Ա���ܼ�ʱ���ֲ�������
�������������������������������ʵķ����϶����������������Ҳ������������ֽ�Ĵ�����?
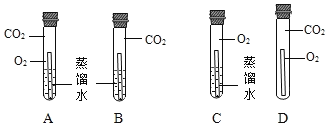
��ʵ��̽����
ʵ�鲽�� | ʵ������ |
I.�ֱ���ȡ5mL5%����������Һ����A��B��֧�Թ��У���A�Թ��м���ag��������ĩ�����ֱ���A��B��֧�Թ��в��������ľ�����۲����� | A�Թ��в������ݣ�������ľ����ȼ,B�Թ������������� |
��.��A�Թ���û��������ʱ�����¼������������Һ�����Ѵ����ǵ�ľ�������Թܣ���˷���������飬�۲����� | ____________________ |
��.��ʵ����е�ʣ����С�Ĺ��ˣ�����������������ϴ�ӡ�������������ù���������Ϊag�� |
��ʵ����ۣ�������������������ֽ�Ĵ�����
�����۽�����
��1��ʵ����Ŀ����____________________;
��2���Թ�A�з���ʽ����ʽΪ____________________��
��ʵ�鷴˼������˿�������е�ȼ��ʵ��ʱ�����ּ���ƿը���ˣ����ܵ�ԭ����_______________��
����Ŀ��ijʵ��С��ͬѧ�ü����������ȡһ������������ȡһ���������������������̻�ϣ���������Ϊ6g�����μ��ȸû����t1��t2��t3��t4ʱ��ֱ���ȴ����ʣ�������������¼���й��������±�����֪�μӻ�ѧ��Ӧ�ĸ����ʵ�����֮�͵��ڷ�Ӧ�����ɵĸ����ʵ�����֮�ͣ���
����ʱ�� | t1 | t2 | t3 | t4 |
ʣ�����������g�� | 4.24 | 4.16 | 4.08 | 4.08 |
��1��д�������������ȡ���������ֱ���ʽ_________________��
��2���Լ�����ȫ��Ӧ�������������������_______ g������д������̣�