��Ŀ����
Ϊ�˲ⶨij������ʯ��������������������ȡ��ʯ��Ʒ40g���������ᣬǡ����ȫ��Ӧʱ������ȥ����219һ���̷���������ҩˮ�Ⱥ����ͷ����ʹͷ����ɲ���״����һ��ҩˮ�Ǽ������ʣ��ڶ������������ʡ�Ϊ���о���Һ�������ǿ���Է��ʵ�Ӱ��̶ȣ�С������ʵ�飬�������£�
g�����ˡ�ϴ�ӡ�����������8g(��ʯ�е����ʼȲ�����ˮҲ�������ᷴӦ)�����㣺
(1)������ʯ��������������������
(2)�������������������
(3)������Һ����������������
g�����ˡ�ϴ�ӡ�����������8g(��ʯ�е����ʼȲ�����ˮҲ�������ᷴӦ)�����㣺
(1)������ʯ��������������������
(2)�������������������
(3)������Һ����������������
��1��80% ��2��20% ��3��25.9%
��1��������������Ϊ��ʯ������������������֮�Ϊ40g-8g=32g
������ʯ������������������="32g/" 40g ��100%=80%
��2����μӷ�Ӧ����������ʵ�����Ϊx�������Ȼ���������Ϊy
6HCl+Fe2O3=2FeCl3+3H2O
219 160 325
x 32g y
219/ x ="160/" 32g �⣬�� x=43.8g��
160/ 32g ="325" /y �⣬�� y=65g
�������������="43.8g" /219g ��100%=20%
(3)������Һ��������������Ϊ
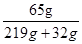 ��100%��25.9%
��100%��25.9%
�𣺣�1��������ʯ������������������Ϊ80%��
(2)�����������������Ϊ20%��
(3)������Һ��������������Ϊ25.9%��
������ʯ������������������="32g/" 40g ��100%=80%
��2����μӷ�Ӧ����������ʵ�����Ϊx�������Ȼ���������Ϊy
6HCl+Fe2O3=2FeCl3+3H2O
219 160 325
x 32g y
219/ x ="160/" 32g �⣬�� x=43.8g��
160/ 32g ="325" /y �⣬�� y=65g
�������������="43.8g" /219g ��100%=20%
(3)������Һ��������������Ϊ
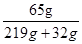 ��100%��25.9%
��100%��25.9%�𣺣�1��������ʯ������������������Ϊ80%��
(2)�����������������Ϊ20%��
(3)������Һ��������������Ϊ25.9%��

��ϰ��ϵ�д�
�����Ŀ
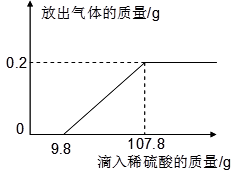
 CaO+CO2������Ʒ�е����ʲ��μӷ�Ӧ���ݴ˼��㣺
CaO+CO2������Ʒ�е����ʲ��μӷ�Ӧ���ݴ˼��㣺