��Ŀ����
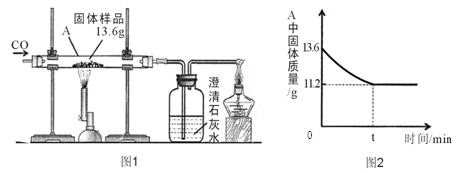
��7�֣�Ϊ�ۺ��������������еĸ���ƷCaSO4��ij����������������Ʊ�(NH4)2SO4�Ĺ������̣�

��1���������У��ڳ����ط����Ļ�ѧ��ӦΪCO2+2NH3+CaSO4+H2O==CaCO3��+ (NH4)2SO4
������¯�з����Ļ�ѧ��Ӧ����ʽΪ ���ù����п�ѭ��ʹ�õ�XΪ (�ѧʽ)������ƷY�к���; (��һ�ּ���)��
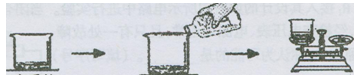
��2������Һ���л��(NH4)2SO4����Ҫ���в���b������b�� ��������һ�����������Һ���У��γ�30��ʱ(NH4)2SO4�ı�����Һ�����ʱ������������Ϊ ����֪30��ʱ����淋��ܽ��Ϊ78g����
��3�����Ʊ�6.6t (NH4)2SO4���壬��������ҪCaSO4���ٶ֣�

��1���������У��ڳ����ط����Ļ�ѧ��ӦΪCO2+2NH3+CaSO4+H2O==CaCO3��+ (NH4)2SO4
������¯�з����Ļ�ѧ��Ӧ����ʽΪ ���ù����п�ѭ��ʹ�õ�XΪ (�ѧʽ)������ƷY�к���; (��һ�ּ���)��
��2������Һ���л��(NH4)2SO4����Ҫ���в���b������b�� ��������һ�����������Һ���У��γ�30��ʱ(NH4)2SO4�ı�����Һ�����ʱ������������Ϊ ����֪30��ʱ����淋��ܽ��Ϊ78g����
��3�����Ʊ�6.6t (NH4)2SO4���壬��������ҪCaSO4���ٶ֣�
��1��CaCO3 CaO+CO2�� CO2 ʳƷ�������
CaO+CO2�� CO2 ʳƷ�������
��2������ 43.8%
��3��6.8��
 CaO+CO2�� CO2 ʳƷ�������
CaO+CO2�� CO2 ʳƷ������� ��2������ 43.8%
��3��6.8��
�����������1������¯�е�������̼��ƣ���ѧ��Ӧ����ʽΪ��CaCO3
 CaO+CO2�����ù����п�ѭ��ʹ�õ�XΪCO2�����и���ƷY�������ƣ���;�ǣ�ʳƷ�������
CaO+CO2�����ù����п�ѭ��ʹ�õ�XΪCO2�����и���ƷY�������ƣ���;�ǣ�ʳƷ���������2������Һ���л��(NH4)2SO4����Ҫ���в���b������b�ǣ�������������һ�����������Һ���У��γ�30��ʱ(NH4)2SO4�ı�����Һ����֪30��ʱ����淋��ܽ��Ϊ78g�����Դ�ʱ������������Ϊ=78g/178g��100%=43.8%
��3�����ݻ�ѧ����ʽ��CO2+2NH3+CaSO4+H2O==CaCO3��+ (NH4)2SO4��(NH4)2SO4��CaSO4��������ϵ���������CaSO4������
�⣺��CaSO4������Ϊx
CO2+2NH3+CaSO4+H2O==CaCO3��+ (NH4)2SO4
136 132
x 6.6t
136��132=x��6.6t
x=6.8t

��ϰ��ϵ�д�
�����Ŀ