ΧβΡΩΡΎ»ί
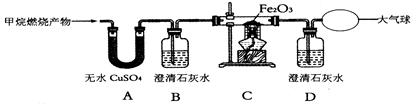
“ΜΧλΘ§–ΓΟςΉΏΫχ Β―ι “Θ§Ω¥ΒΫΝΥ“ΜΖυΓΑ≤ΜΚΆ–≥Γ±ΒΡΜ≠ΟφΘ®»γΆΦΘ©Θ§Έß»Τ¥ΥΤΩ ‘ΦΝ «Ζώ±δ÷ ΒΡΈ ΧβΘ§’ΙΩΣΝΥΧΫΨΩΓΘ
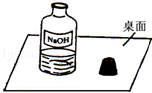
Θ®1Θ©–ΓΟςΧα≥ωœ¬Ν–≤¬œκΘΚ
≤¬œκ“ΜΘΚ»γΙϊ ‘ΦΝΆξ»Ϊ±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ « ΘΜ
≤¬œκΕΰΘΚ»γΙϊ ‘ΦΝ≤ΩΖ÷±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ «NaOHΚΆNa2CO3ΘΜ
≤¬œκ»ΐΘΚ»γΙϊ ‘ΦΝΟΜ”–±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ «NaOHΓΘ
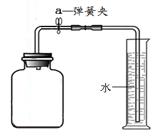
Θ®2Θ©–¥≥ωNaOHΖΔ…ζ±δ÷ ΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚ___________________________________ΓΘ
Θ®3Θ©«κΡψ…ηΦΤ Β―ι÷Λ ΒΗΟ ‘ΦΝ“―Ψ≠≤ΩΖ÷±δ÷ Θ§Άξ≥…ΧΫΨΩΖΫΑΗΘΚ

Θ®1Θ©–ΓΟςΧα≥ωœ¬Ν–≤¬œκΘΚ
≤¬œκ“ΜΘΚ»γΙϊ ‘ΦΝΆξ»Ϊ±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ « ΘΜ
≤¬œκΕΰΘΚ»γΙϊ ‘ΦΝ≤ΩΖ÷±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ «NaOHΚΆNa2CO3ΘΜ
≤¬œκ»ΐΘΚ»γΙϊ ‘ΦΝΟΜ”–±δ÷ Θ§‘ρ»ή“Κ÷–»ή÷ «NaOHΓΘ
Θ®2Θ©–¥≥ωNaOHΖΔ…ζ±δ÷ ΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚ___________________________________ΓΘ
Θ®3Θ©«κΡψ…ηΦΤ Β―ι÷Λ ΒΗΟ ‘ΦΝ“―Ψ≠≤ΩΖ÷±δ÷ Θ§Άξ≥…ΧΫΨΩΖΫΑΗΘΚ
| Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσΦΑΫα¬έ |
| | |
| | |
Θ®1Θ©Na2CO3Θ®ΜρΧΦΥαΡΤΘ©ΘΜΘ®2Θ©2NaOH+CO2®TNa2CO3+H2OΘΜΘ®3Θ©
| Β―ι≤Ϋ÷η | ‘ΛΤΎœ÷œσΦΑΫα¬έ |
| »Γ…ΌΝΩ¥ΐ≤β“Κ”Ύ ‘ΙήΘ§Φ”»κΉψΝΩBaCl2»ή“Κ | ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§‘ρΥΒΟς»ή“Κ÷–Κ§”–Na2CO3 |
| œρΨ≤÷ΟΚσΒΡ…œ ω ‘Ιή÷–Θ§ΒΈΦ”…ΌΝΩΖ”ΧΣ»ή“Κ | »ή“Κ±δΚλΘ§‘ρΥΒΟς»ή“Κ÷–Κ§”–NaOH |
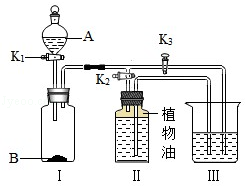
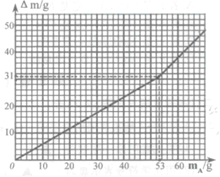
‘ΧβΖ÷ΈωΘΚΘ®1Θ©«β―θΜ·ΡΤ»ή“Κ»τΈϋ ’Ω’Τχ÷–ΒΡΕΰ―θΜ·ΧΦΤχΧεΜα…ζ≥…ΧΦΥαΡΤΕχ±δ÷ Θ§¥Υ ±”–ΝΫ÷÷Ω…ΡήΘΚ“Μ «≤ΩΖ÷±δ÷ Θ®»ή÷ «NaOHΚΆNa2CO3Θ©Θ§Εΰ «»Ϊ≤Ω±δ÷ Θ®»ή÷ «Na2CO3Θ©ΘΜ
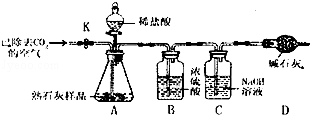
Θ®2Θ©«β―θΜ·ΡΤ±δ÷ «Έϋ ’Εΰ―θΜ·ΧΦ≤ζ…ζΧΦΥαΡΤΚΆΥ°Θ§Ι Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣΘΚ2NaOH+CO2ΘΫNa2CO3+H2OΘΜ
Θ®3Θ©Ζά≥ΐ»ΞΧΦΥαΡΤΕ‘«β―θΜ·ΡΤΦλ―ιΒΡ”ΑœλΘ§Ω…≤…»ΓΦ”»κΉψΝΩ¬»Μ·±ΒΘ®Μρ¬»Μ·ΗΤΘ©»ή“ΚΒΡΖΫΖ®Α―ΧΦΥαΡΤ»Ϊ≤ΩΉΣ±δ≥…≥ΝΒμΘ§‘ΌΦ”»κΖ”ΧΣ ‘“Κ“‘Φλ―ι»ή“Κ÷–ΜΙΚ§”–«β―θΜ·ΡΤΘ§Ι ¥πΑΗΈΣΘΚ
Β―ι≤ΌΉς Β―ιœ÷œσΦΑΫα¬έ
»Γ…ΌΝΩ¥ΐ≤β“Κ”Ύ ‘ΙήΘ§Φ”»κΉψΝΩBaCl2»ή“Κ ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§‘ρΥΒΟς»ή“Κ÷–Κ§”–Na2CO3
œρΨ≤÷ΟΚσΒΡ…œ ω ‘Ιή÷–Θ§ΒΈΦ”…ΌΝΩΖ”ΧΣ»ή“Κ »ή“Κ±δΚλΘ§‘ρΥΒΟς»ή“Κ÷–Κ§”–NaOH

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ