��Ŀ����
28.��6�֣�����кͷ�Ӧ��ʵ���������������Ź㷺��Ӧ�ã��磺������������������θ�����ȡ�
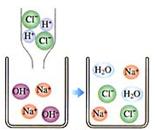
С��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�����һ�� �����������˵μ�ָʾ����Ϊ��ȷ�����������������Ƿ�ǡ����ȫ��Ӧ��С�����ձ���ȡ������Ӧ�����Һ�����Թ��У������Թ��еμ�2����ɫ��̪�����۲쵽��̪�Լ�����ɫ���������ó�������������ǡ����ȫ�к͡��Ľ��ۡ�
��1������Ϊ���ó��Ľ��� (���ȷ�����
������ ��
(2)��ͼ��С��ͬѧ���Ƴ�������кͷ�Ӧ����ʾ��ͼ���÷�Ӧ
�Ļ�ѧ����ʽΪ ��
��3�����������ʵ�飬̽�������ձ��е���Һ�Ƿ�ǡ����ȫ�к͡�
| ʵ�鲽�� | ʵ������ | ���� |
|
|
|
|
��6�֣�
��1������ ������������̪�Լ�Ҳ����ɫ
��2��NaOH+HCl=NaCl+H2O
��3��
| ʵ�鲽�� | ʵ������ | ���� |
| ���ձ���ȡ������Ӧ�����Һ�����Թ��У������Թ��еμ�2����ɫʯ����Һ�����۲� | ��ɫʯ����Һ��ɺ�ɫ | û����ȫ�кͣ�������� |

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�
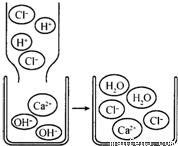
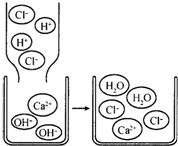 ��2008?��������ģ�������������ʵģ��������������ӹ��ɵġ�����ѧ�仯��ʵ��������������ϵĹ��̡��ȶ��ǻ�ѧѧ���е���Ҫ�۵㣮���ݸ���Ԫ�صĻ��ϼۣ���
��2008?��������ģ�������������ʵģ��������������ӹ��ɵġ�����ѧ�仯��ʵ��������������ϵĹ��̡��ȶ��ǻ�ѧѧ���е���Ҫ�۵㣮���ݸ���Ԫ�صĻ��ϼۣ��� ����кͷ�Ӧ��ʵ���������������Ź㷺��Ӧ�ã��磺������������������θ�����ȣ�С��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ������ͼ1���ձ��е�����������Һ�μ�����һ�ᣬ���������˵μ�ָʾ����Ϊ��ȷ�����������������Ƿ�ǡ����ȫ��Ӧ��С�����ձ���ȡ������Ӧ�����Һ�����Թ��У������Թ��еμ�2����ɫ��̪�����۲쵽��̪�Լ�����ɫ���������ó�������������ǡ����ȫ�к͡��Ľ��ۣ�
����кͷ�Ӧ��ʵ���������������Ź㷺��Ӧ�ã��磺������������������θ�����ȣ�С��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ������ͼ1���ձ��е�����������Һ�μ�����һ�ᣬ���������˵μ�ָʾ����Ϊ��ȷ�����������������Ƿ�ǡ����ȫ��Ӧ��С�����ձ���ȡ������Ӧ�����Һ�����Թ��У������Թ��еμ�2����ɫ��̪�����۲쵽��̪�Լ�����ɫ���������ó�������������ǡ����ȫ�к͡��Ľ��ۣ�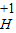 ��
�� ��
��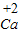 ��
��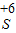 ��
�� ��
�� ��
�� ����Ԫ������ѡһ�ֻ���Ԫ��������ʣ��ش��������⣮
����Ԫ������ѡһ�ֻ���Ԫ��������ʣ��ش��������⣮