��Ŀ����
����Ŀ��ʵ����������5 g��������Ϊ50����NaCl��Һ���Իش��������⣮
(1)��ʵ��IJ���������_________��_________��_________��
(2)�ֱ�˵����ͼ��A��B������ʵ������Ӱ�죬��������

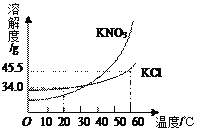
A��������������Һ���ʵ���������_______(�ƫ��ƫС�����䡱)��������_____����������������ȷ��A������������������������Ϊ_______%��
B��������������Һ��������������_______(�ƫ��ƫС�����䡱)��������_____��
���𰸡� ���� ���� �ܽ� ƫС �������� 37��5 ƫ�� ����Ӧ�밼Һ����ʹ���ƽ
�������������������������Ĺ�ʽ���㣬����5 g ������������Ϊ50% ���Ȼ�����Һ��Ҫ�Ȼ��Ƶ�����Ϊ5g ��50% =2.5 g����Ҫˮ������Ϊ5 g-2.5 g=2. 5 g��A ������������Ȼ��Ƶ�λ�õߵ��ˣ�ʵ�ʳƵ��Ȼ��Ƶ�����Ϊ2 g-0. 5 g=1.5 g������������Һ��������������ƫС��B �������ɼ���֪��Ҫ��ȡ2.5 mL ��ˮ����ʵ�ʲ���ʱ���Ӷ���������ȡ��ˮ����2.5 mL���ʵ���������Һ��������������ƫ��
�⣺(1)��ʵ��IJ��������ǣ����㡢�������ܽ⣻
(2) A��������������Һ���ʵ���������ƫС ���������������룻��������������ȷ��A������������������������Ϊ![]() =37��5����B��������������Һ��������������ƫ����������Ӧ�밼Һ����ʹ���ƽ��
=37��5����B��������������Һ��������������ƫ����������Ӧ�밼Һ����ʹ���ƽ��

 ��ɢ˼ά�¿���ϵ�д�
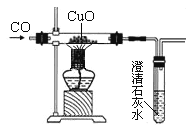
��ɢ˼ά�¿���ϵ�д�����Ŀ����֪����ͭ��ԭ��ͭ�Ĺ�������������ͭ���ɡ���ͼ��С��ͬѧ��CO��ԭ����ͭ��ʵ��װ��ͼ�����������к�ɫ����ȫ������С����֪����ɫ����������Щ�ɷ֣�������С��һ����������̽����
��������롿��ȫ����ͭ�� ��ȫ����������ͭ������ͭ��������ͭ�Ļ���
���������ϡ�������ͭ��һ�ֺ�ɫ���壬����ϡ���ᷢ�����·�Ӧ��
Cu2O+H2SO4=Cu+CuSO4+H2O
�����ʵ�顿Ϊ����֤��Щ���룬С��ͬѧ����������̽�����������������⣺
���� | ��������� | ���� |
��1����ȡ14.4g��Ӧ���ɵĺ�ɫ�������Թ��У������м���������ϡ��� | ����_______�� | ����ٴ��� |
��2�������裨1����Ӧ������ʽ��й��ˣ����������Ĺ�������Ϊm�� | ���ݣ� ��m = 6.4g�� | ________ |
���ݣ���m_______6.4g (���������)�� | �������ȷ |
��������˼����1��С��ʵ��װ���е�ʯ��ˮ����Ҫ������______________________________���ӻ����Ƕȿ�����װ���д��Ľ�����д��һ�ָĽ���ʩ_____________________________��
��2����д������ͭ��CO��Ӧ����������ͭ�Ļ�ѧ����ʽ_________________________��