��Ŀ����
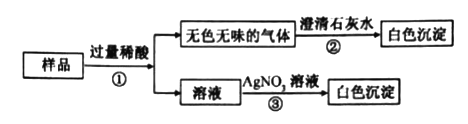
����Ŀ��ij��Ʒ�������к����Ȼ�������.ѧУ��ѧ��ȤС���ͬѧ��չ�˲ⶨ��������������������̽��ʵ��.������������µ�ʵ�鷽����

��1��������ʹ�õ��������������� ��
��2������Ӧ��ϴ�Ӻ��ɳ����������ʹ�ⶨ�Ľ�� ���ƫ��ƫС�����䡱����
��3��Ϊ�˼�������Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ�Ӻ��Һ���������Թ��У��ٵμ� ��Һ��������������˵��������ϴ�Ӹɾ���
��4������Ʒ�������Ƶ����������Ƕ��٣���д��������̣�
���𰸡�������ƫ����������Һ��71%
����������1��������ʹ�õ�����������������������2������Ӧ��ϴ�Ӻ��ɳ����������ʹ�ⶨ�Ľ��ƫ��3��Ϊ�˼�������Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ�Ӻ��Һ���������Թ��У��ٵμ���������Һ��������������˵��������ϴ�Ӹɾ���
��4����������Ʒ�������Ƶ�����������x
BaCl2 ��Na2SO4 ��Ba SO4����2NaCl
142 233
20g��x 23.3g
![]() ��
��![]() ,x��71��.
,x��71��.
��������Ʒ�������Ƶ�����������71����
�㾦��������Ҫ����ʵ��Ļ��������Լ����ݻ�ѧ����ʽ���м��㡣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ