ƒøƒ⁄»ð
°æƒø°øÕÍ≥…œ¬¡–∑¥”¶µƒªØ—ß∑Ω≥Ã Ω£¨≤¢ªÿ¥”–πÿŒ °£
£®1£©π§“µ…œ”√≥ýÃ˙øÛ‘⁄∏þŒ¬Ãıº˛œ¬¡∂Ã˙µƒ‘≠¿Ì£∫
£¨“±¡∂…˙Ã˙µƒ…˱∏ « £ª
£®2£©ºÏ—È∂˛—ıªØú£¨Ω´∂˛—ıªØú∆¯ÃÂÕ®»Î±•∫Õ Øª“ÀÆ£∫
£¨∑¥”¶∫ۻГ∫ (—°ÃÓ°∞±•∫Õ°±ªÚ°∞≤ª±•∫Õ°±£©£ª
£®3£©¬»ªØÔßæßÃÂ∫Õ Ï Øª“ªÏ∫œ—–ƒ•ª·…˙≥…“ª÷÷∞±≥ÙŒ∂µƒ∆¯Ã£∫
£¨ºÏ—È∏√∆¯ÃÂø…”√ ‘÷Ω£ª
£®4£©”√∞±ÀÆ÷–∫Õ¡ÚÀ·≥ß≈≈≥ˆ¿¥µƒ∑œÀÆ£∫
£¨π˝≥Ã÷–∑œÀƵƒpH (—°ÃÓ°∞±‰¥Û°±ªÚ°∞±‰–°°±£©£ª
£®5£©√¿æÁ°∂‘Ω”¸°∑÷–¬ıøÀŒ™¡À”√¡ÚÀ·∏Ø ¥“ΩŒÒ “œ¬ÀƵ¿”√”⁄Ô…˙£¨”⁄ «”√¡◊À·∫Õ¡ÚÀ·∏∆∑¥”¶÷∆µ√¡◊À·∏∆≥¡µÌ∫Õ¡ÚÀ·£∫
£¨’‚∏ˆ∑¥”¶ Ù”⁄ ∑¥”¶¿ý–Õ°£
°æ¥∞∏°ø£®1£©Fe2O3 +3CO![]() 2Fe+3CO2∏þ¬Ø£®2£©Ca£®OH£©2+CO2===H2O+CaCO3°˝£ª≤ª±•∫Õ
2Fe+3CO2∏þ¬Ø£®2£©Ca£®OH£©2+CO2===H2O+CaCO3°˝£ª≤ª±•∫Õ
£®3£©Ca£®OH£©2+2NH4Cl===CaCl2+2NH3°¸+2H2O;¿∂…´µƒ Ø»Ô ‘÷Ω£®4£©H2SO4 + 2NH3.H2O ==£® NH4£©2SO4 + 2H2O£ª±‰¥Û£®5£©2H3PO4 + 3Ca SO4 ==Ca3(PO4£©2 °˝+ 3H2SO4£ª∏¥∑÷Ω‚∑¥”¶
°æΩ‚Œˆ°ø
‘Â∑÷Œˆ£∫—ߪ·’˝»∑µƒ∂˛ ¶–÷ªØ—ß∑Ω≥Ã Ω£¨ªπ“™∫œ¿Ìµƒ±Í◊¢∑¥”¶µƒÃıº˛∫Õ’˝»∑µƒ≈‰∆ΩªØ—ß∑Ω≥Ã Ω£¨∏˘æðªØ—ß∑Ω≥Ã Ω¿¥≈–∂œ∑¥”¶µƒ¿ý–Õ°£

°æƒø°ø—ıªØ√æ‘⁄“Ω“©°¢Ω®÷˛µ»––“µ”¶”√π„∑∫°£“‘¡‚√æøÛ(÷˜“™≥…∑÷Œ™MgCO3£¨∫¨…Ÿ¡øFeCO3£¨∆‰À˚‘”÷ ≤ª»Ð”⁄À·)Œ™‘≠¡œ÷∆±∏∏þ¥ø—ıªØ√浃 µ—È¡˜≥ûÁœ¬£∫
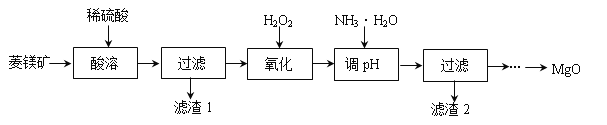
£®1£©À·»Ð÷Æ«∞“™Ω´øÛ Ø∑€Àȵƒƒøµƒ « °£œ°¡ÚÀ·–Ë“™π˝¡øµƒƒøµƒ « °£À·»Ð≤Ω÷Ë÷–úÀ·√æ»ÐΩ‚µƒ∑¥”¶∑Ω≥Ã ΩŒ™ °£
£®2£©“—÷™Mg2+°¢Fe2+∫ÕFe3+‘⁄»Ð“∫÷–”ˆµΩ∞±ÀÆæ˘ø…“‘◊™ªØ≥…ƒ—»Ð–‘ºÓ≥¡µÌ£¨ø™ º≥¡µÌ∫Õ≥¡µÌÕÍ»´µƒpH»Áœ¬±Ì£∫
Mg2+ | Fe2+ | Fe3+ | |
ø™ º≥¡µÌ | 9.4 | 7.9 | 2.7 |
≥¡µÌÕÍ»´ | 12.4 | 9.6 | 3.7 |
¡˜≥ð∞—ıªØ°±“ª≤Ω÷–H2O2µƒ◊˜”√ «Ω´»Ð“∫÷–µƒFe2+◊™ªØ≥…Fe3+£¨≤ªƒÐ÷±Ω”≥¡µÌFe2+µƒ‘≠“Ú « °£
£®3£©º”∞±ÀƵ˜Ω⁄»Ð“∫µƒPH∑∂ŒßŒ™ °£
£®4£©¬À‘¸2µƒªØ—ß Ω « °£
°æƒø°ø«Îƒ„Ω·∫œœ¬¡–◊∞÷√Õºªÿ¥Œ £∫
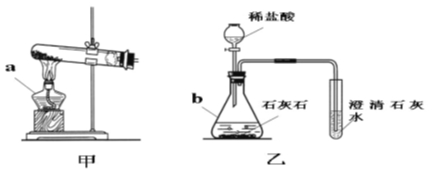
£®1£©–¥≥ˆ”–±Í∫≈“«∆˜µƒ√˚≥∆£∫a____________£¨b____________°£
£®2£© µ—È “”√º◊◊∞÷√÷∆—ı∆¯µƒªØ—ß∑Ω≥Ã Ω «_________________________________£¨”√____________∑® ’ºØ—ı∆¯°£∑¥”¶Ω· ¯∫Û¿‰»¥£¨Õ˘ ‘πÐ÷–º”»Î◊„¡øµƒÀÆ£¨Ω¡∞Ë°¢π˝¬À£¨µ√µΩ∫⁄…´∑€ƒ©°£∏√∫⁄…´∑€ƒ©”Îπ˝—ıªØ«‚Ω”¥•”–¥Û¡ø∆¯≈ð≤˙…˙£¨∑¥”¶µƒªØ—ß∑Ω≥Ã Ω «_______________________£¨∫⁄…´∑€ƒ©‘⁄∑¥”¶÷–µƒ◊˜”√ «_______________°£
£®3£©ƒ≥ªØ—ß–À»§–°◊È”√““◊∞÷√÷∆»°≤¢ºÏ—È∂˛—ıªØú°£ µ—Èπ˝≥Ã÷–£¨ø…π€≤쵽 ‘πпÔ≤˙…˙______…´≥¡µÌ£¨∑¥”¶µƒªØ—ß∑Ω≥Ã Ω «_________________________________°£≤˙…˙≥¡µÌ∫ۺÖ¯Õ®»Î∂˛—ıªØú£¨π˝“ª∂Œ ±º‰∫Û£¨∑¢œ÷≥¡µÌ»ÐΩ‚±‰≥…≥Œ«Â»Ð“∫°£Œ™¡À»∑∂®≥¡µÌ»ÐΩ‚≥…≥Œ«Â»Ð“∫µƒ‘≠“Ú£¨–°◊ȵƒÕ¨—ßΩ¯––¡ÀœýπÿÃΩæø°£
£®Ã·≥ˆŒ £©£∫≥¡µÌŒ™ ≤√¥ƒÐ»ÐΩ‚±‰≥…≥Œ«Â»Ð“∫£ø
£®≤È‘ƒ◊ ¡œ£©£∫úÀ·—Œ»Ð”⁄À·£¨ÃºÀ·«‚∏∆£€Ca£®HCO3£©2£ð»Ð”⁄ÀÆ°£
£®≤¬œÎ”κŸ…Ë£©£∫¢Ÿ»Ð“∫≥ À·–‘£ª¢⁄∑¥”¶…˙≥…¡ÀúÀ·«‚∏∆°£
£® µ—È”ÎΩ·¬€£©£∫
µ—È≤Ÿ◊˜ | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
µ—È¢Ò£∫∞—“ª–°∆¨pH ‘÷Ω∑≈‘⁄“ªøÈ∏…檵ƒ≤£¡ß∆¨…œ£¨”√_________’∫»°≥¡µÌ»ÐΩ‚≥…≥Œ«Âµƒ»Ð“∫’¥‘⁄ ‘÷Ω…œ£¨∞— ‘÷Ω≥ œ÷µƒ—’…´”αÍ◊º±»…´ø®∂‘’’°£ | ≤‚µ√±ª≤‚“∫µƒpH£Ω8 | ≤¬œÎ¢Ÿ______________°£ £®ÃÓ°∞≥…¡¢°±ªÚ°∞≤ª≥…¡¢°±£© |
µ—È¢Ú£∫»°≥¡µÌ»ÐΩ‚≥…≥Œ«Âµƒ»Ð“∫”⁄¡Ì“ª÷ß ‘πÐ÷–£¨º”»Î_____________________ ___________________°£ | ”–∆¯ÃÂ≤˙…˙ | ∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™£∫ _______________________________°£ ≤¬œÎ¢⁄≥…¡¢°£ |
Õ®π˝ÃΩæøµ√÷™£¨…˙≥…µƒ≥¡µÌª·”Î∂˛—ıªØú°¢ÀÆ∑¥”¶…˙≥…¡Àø…»Ð”⁄ÀƵƒÃºÀ·«‚∏∆°£
£®Ωª¡˜”Î∑¥Àº£©£∫
¥”ÃΩæø÷–ƒ„µ√µΩµƒ∆Ù æªÚ∏– Ð « °£
