��Ŀ����
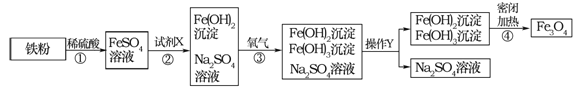
����Ŀ���ں�����������ʱ��Ϊȷ��ʩ�����������̼�����Ա���õ�һ�ֽ��������������ķ�Ӧ���Բ��ϡ�ʵ���ҵ��Ʊ��������£�
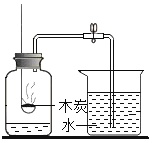
��1���ڸ�������������ͨ���ۺ�ˮ������Ӧ���õ���ĩ״��������������
��2����������������ĩ�ڸ��������¾��������������෴�ķ�Ӧ����������������ĩ����ֱ�����������װ�ã���ȡ������������������������ʵ���б���ʹ����ͨ���ۺ�ϡ���ᣬ�����Լ�����Ʒ��ѡ����ע��װ���б�Ҫ������̨�����С���Ȧ��ʯ�����������豸����ͼ�о�����ȥ��

��д���пհף�
��1��ʵ������еڣ�1������2�������෴����ķ�Ӧ����ʽ�ǣ���______����______��
��2����ʵ��װ���У���ƿB��������______��
��3��ʵ��ʱ�Թ�A�м���Ĺ�����______���Թ�D�����ռ�����������______��
��4��ʵ��ʱ��Һ©��E��Ӧ������Լ���______����F�м���Ĺ�����______����U��G�г���������������ƣ���������______��
��5��ʵ������У�����װ����������Ҫ���ȵ�������______������ĸ����
���𰸡�3Fe+4H2O![]() 4H2+Fe3O4 Fe3O4+4H2
4H2+Fe3O4 Fe3O4+4H2![]() 3Fe+4H2O ����ˮ���� ���� H2 ϡ���� п�� ��ȥ�����е�ˮ������ A��B��H
3Fe+4H2O ����ˮ���� ���� H2 ϡ���� п�� ��ȥ�����е�ˮ������ A��B��H
��������
��1��������������ṩ�����ʿ����ƶϳ���һ��ʵ���г�������������������������������ʱ���Ĵ�Ϊ��
3Fe+4H2O![]() 4H2+Fe3O4��Fe3O4+4H2
4H2+Fe3O4��Fe3O4+4H2![]() 3Fe+4H2O
3Fe+4H2O
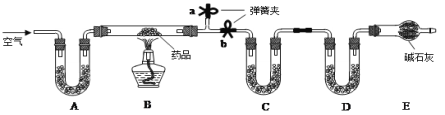
��2����ƿ�����þ��Ǽ���Һ����߶�Һ���������������������ƿ��������þ��Dz���ˮ������Ϊ�˷�ֹ���Թ���ͨ��ˮ����ʹ�Թ����䣬����ˮ�ĵ����Ӷ����Թܺ��ռ�������Թ��м�����˹��ƿ���Ա�֤ʵ�鰲ȫ��
��3���Թ�A������ˮ������Ӧ��װ�ã�����A��װ��Ӧ������ͨ���ۣ��ڷ�Ӧ������ Ҫ��������������D�������ռ������ġ�
��4��װ��F��������ȡ������װ�ã�����װ��E����������ϡ����ģ�����ʹ�õ���ϡ��������ȡ�����������ڲ���������ͬʱ���ܴ�������ˮ����������U���м��������������������塣
��5����Ӧ���ڸ��µ������·�Ӧ�ģ�����A��Hװ��Ҫ���ȣ�ͬʱ��Ӧ��Ҫˮ�����IJμӣ���BҲ��Ҫ���ȡ�
�ʴ�Ϊ����1��3Fe+4H2O![]() Fe3O4+4H2��Fe3O4+4H2
Fe3O4+4H2��Fe3O4+4H2![]() Fe+4H2O��2������ˮ������3�����ۣ�H2��4��ϡ���п������ȥ�����е�ˮ������
Fe+4H2O��2������ˮ������3�����ۣ�H2��4��ϡ���п������ȥ�����е�ˮ������
��5��A��B��H