��Ŀ����
����Ŀ��Ϊ�˲������⣨Fe2O3��xH2O��������������������������ʵ�飺
����٣�ȡ20g���⣬����������14.6%��ϡ����200g����ַ�Ӧ��
����ڣ���ٷ�Ӧ�����Һ����������16%��NaOH��Һ����ҺpH�ı仯��ͼ��ʾ��

��1������ٷ�Ӧ��������Һ�е������� ��д��ѧʽ����
��2�����������FeCl3��Ӧ��NaOH��Һ�������� g�����ɳ����������Ƕ��٣���д��������̣���ȷ��0.1��
��3�����ݼ�������������ͼ�л����������������ߡ�

��4��������������������Ϊ ����ȷ��0.1������
���𰸡���1��FeCl3��HCl����2��150g�����ɳ���������Ϊ21.4g����3��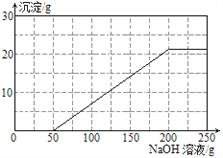 ��
��
��4��56%
����������1�������������ᷴӦ�����Ȼ�����ˮ�������������������Է�Ӧ������ΪFeCl3��HCl��
��2�������������������ᷴӦ��ʹ��Һ��pHֵ���Ϊ7�������������Ȼ�����Ӧʱ��Һ��pHֵ���䣬������FeCl3��Ӧ��NaOH��Һ��������200g-50g=150g��
�����ɳ�����������x
FeCl3+3NaOH�TFe��OH��3��+3NaCl��
120 107
150g��16% x
![]()
x=21.4g
�����ɳ���������Ϊ21.4g��
��3�����Բ�������������Ϊ ��
��
��4������������������������Ϊ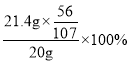 =56%��
=56%��

����Ŀ���±��г��˹�������A�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
�¶ȣ��� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
�ܽ�ȣ�g | 36 | 35 | 34 | 33 | 32 | 31 | 30 | 29 | 28 | 27 |
(1)70��ʱ����ʢ��100gˮ���ձ��м���30g����A������ܽ��γɵ���______________(����͡������͡�)��Һ���ٽ��ձ��������¶Ƚ���20�棬��ʱ��Һ���������ܼ���������Ϊ_____________(�����������)��
(2)ͨ�����ϱ����ݵķ���������A���ܽ������Ӧ����ͼ�е�___________(��ס����ҡ�)��

(3)80��ʱ������һ����A���ʵ���Һ�����併�µ�60�棬�Ƿ��й�������?____________(��С��� ��û�С���ȷ����)��





