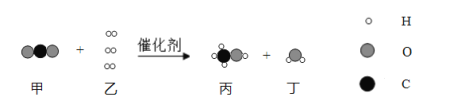
��Ŀ����
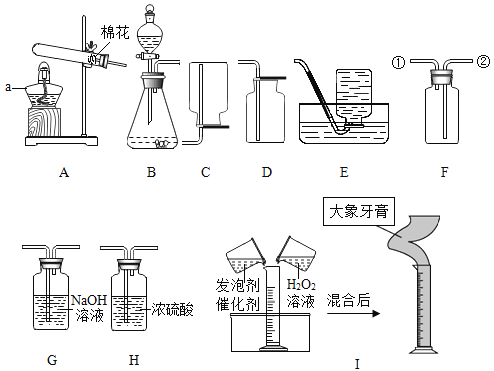
����Ŀ���������������Ĵ��������������⣬ij�����������������������д������ۺ����õIJ���������ͼ��
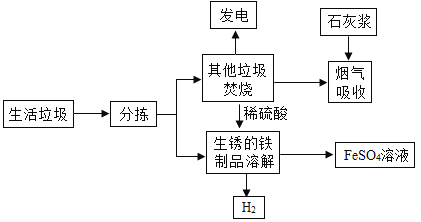
����1���������ղ����������к���SO2��HCl���к�����
����2��ʯ�ҽ�����Ҫ�ɷ�����������
����3��+2�۵���Ԫ�����ױ������е�����������
�ش��������⣺
��1�����ղ����У�ʯ�ҽ���������_____��
��2���ܽⲽ���У�������H2�Ļ�ѧ����ʽΪ_____��
���˷�Ӧ�⣬�ܽⲽ�軹�ܷ���������Ӧ��
��Fe2O3+3H2SO4�TFe2��SO4��3+3H2O
��Fe+Fe2��SO4��3�T3FeSO4��
��3������������������Һ�ڵ��������м�������Ũ������ȴ�ᾧ��_____����������ƣ����õ������������壬���е�����������_____��
��4�������������ͷ���ճ���������������һ�����飺_____��
���𰸡���ȥ�����к��е�SO2��HCl Fe+H2SO4�TFeSO4+H2�� ���� ������������+2�۵���Ԫ�ر������е��������� ��������������յ�
��������
��1��ʯ�ҽ��Լ��ԣ��ܹ������������壬�������ղ����У�ʯ�ҽ��������dz�ȥ�����к��е�SO2��HCl��
��2���ܽⲽ���У�����������Ӧ���������������Բ�����H2�Ļ�ѧ����ʽΪFe+H2SO4�TFeSO4+H2����
��3�������ڵ�Ŀ���ǵõ������������壬�����������裺����Ũ����Ȼ����ȴ�ᾧ���ٹ��ˣ����ϴ�Ӹ���õ������������壬���е�������������������������+2�۵���Ԫ�ر������е�����������
��4��������������������յȣ��������ɣ���