��Ŀ����
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
I��ʵ�鷽��
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH����õ�pH��Сֱ��pHС��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����Ӧ��������Һ��______�ԣ���ᡱ��������С���
��2����pH��ֽ�ⶨNaOH��ҺpHʱ����ȷ�IJ����ǣ�______��
��3������ǿ������õ�pHС��7�������ɣ�______��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10�˻�ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
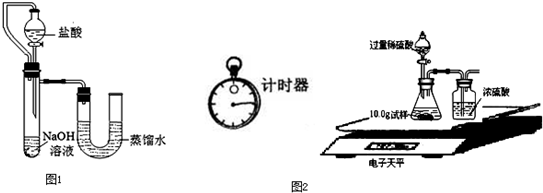
��5��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ��ͼ1��ʾװ�������ʵ�飮���ͬѧ����ʵ������______���ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
II��ʵ���е��������
��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3��
��6��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м���������______��Һ��������ɫ����������Ȼ�����ϲ���Һ�м����̪��Һ��������Һ�ʺ�ɫ����֤�˲��������ȷ�ģ�
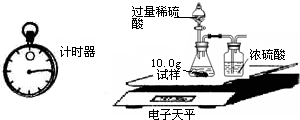
��7��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ����ͼ��ʾ��ʵ�飮
ʵ�����ݼ�¼���£�
| �ơ� ���� � Ŀ | �ơ� ����ʱ �� | ������g�� |
| ���� | 10.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 249.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 249.00 |
��8����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ����______��
�⣺����һ��
��1����õ�pH��Сֱ��pHС��7��˵��������Һ�����ԣ�
�ʴ�Ϊ���
��2����pH��ֽ�ⶨ����������Һ��pHʱ����ȷ�IJ����ǣ��ø���IJ�����պһ��NaOH��Һ���ڸɾ��İ״ɰ����Ƭ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó���Һ��pH��
�ʴ�Ϊ���ø���IJ�����պһ��NaOH��Һ���ڸɾ��İ״ɰ����Ƭ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó���Һ��pH��
��3���Ʋ⡰��õ�pHС��7�������ɣ��ų�������ļ��룬ϡ������������Һ������pH��С��
�ʴ�Ϊ��
�ų���ϡ�Ͷ�ʹ��ҺpH��С�����أ�
����������
��4����ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ������ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t��
�������������Ũ�ȶ��ǵ�һ���2�������������ǵ�һ���4�����¶ȱ仯���ǵ�һ���4���������������������ǵڶ����2���������¶ȵĸı�Ҳ�ǵ������ �����Ƴ�x=7��
�����Ƴ�x=7��
�ʴ�Ϊ��7��
��5���������ƺ����ᷢ���кͷ�Ӧ����Һ���¶Ȼ����ߣ�������ƿ�ڵ�ѹǿ����ʹU�ιܵ�Һ����ָ߶Ȳijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����U�ι������Һ���½���ӳ��Һ���Ȳ��ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
�ʴ�Ϊ��
U�ι������Һ���½���
��6��Ҫ��֤������Ƿ���ȷ����Ҫ�Ǽ���̼�������������ƵĴ��ڣ�һ�������ö�̼����ļ����Լ�������Һ�ļ�����ɣ���˼�����λ����Լ���̼��������ܼ����Ӧ�ļ��ֹ���ţ������̪���������Ƶļ��Խ��з����жϣ�
�ʴ�Ϊ��CaCl2��BaCl2��
��7�������������ݿ�֪����Һ�ڷ�Ӧǰ�����ʵ������仯�������Ƿ�Ӧ���ɵĶ�����̼�������ݴ˿����ݷ���ʽ���̼���Ƶ�������Ҳ��֪�������Ƶ�������
������̼�������ǣ�241.20g+10.00g-249.00g=2.2g
����Ʒ��̼���Ƶ�����Ϊx
H2SO4+Na2CO3=Na2SO4 +H2O+CO2��
106 44
x 2.2g
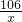 =
=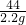
x=5.3 g
Na2CO3����������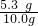 ��100%=53%
��100%=53%
NaOH����������1-53%=47%
��8��������̼���ܶȽϴ����������ײд������Ķ�����̼�����²����Ķ�����̼������С��̼���Ƶ�������С��������������С��
�ʴ�Ϊ��
CO2���ܶȱȿ���������װ���У�������ղ�õ�����ƫ������ɵ�CO2����ƫС����Na2CO3����������ƫС��
����������һ����1��������֪����õ�pH��Сֱ��pHС��7��˵��������Һ�����ԣ�
��2����pH��ֽ�ⶨ��Һ��pHʱ��������ȷ�IJ���������
��3������ǿ������õ�pHС��7�������ɣ�����ϡ�����ϡ�͵����ã�
����������4���ɱ������ݣ����Ƴ�x��ֵ��
��5��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� U�ι������Һ���½��ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
��6����֤������Ƿ���ȷ����Ҫ�Ǽ���̼�������������ƵĴ��ڣ�һ�������ö�̼����ļ����Լ�������Һ�ļ�����ɣ�
��7���������ϵ�֪����Һ�ڷ�Ӧǰ�����ʵ������仯�������Ƿ�Ӧ���ɵĶ�����̼�������ݴ˿����ݷ���ʽ���̼���Ƶ�������Ҳ��֪�������Ƶ�������
��8�����ݶ�����̼���ܶȽϴ�������ɣ�
�����������Ķ����ϴ������кͷ�Ӧ�ĸ����Ӧ�ã�pH��ֽ�ⶨ��Һ��pHʱ�IJ�������������ʽ�����֪ʶ�����������������ߵ����ϵͣ��ڽ���ʱ����Ҫ������Ҫ�������⣬Ȼ��Ӧ�������յ�֪ʶ��һ������ɣ�
��1����õ�pH��Сֱ��pHС��7��˵��������Һ�����ԣ�
�ʴ�Ϊ���
��2����pH��ֽ�ⶨ����������Һ��pHʱ����ȷ�IJ����ǣ��ø���IJ�����պһ��NaOH��Һ���ڸɾ��İ״ɰ����Ƭ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó���Һ��pH��
�ʴ�Ϊ���ø���IJ�����պһ��NaOH��Һ���ڸɾ��İ״ɰ����Ƭ�ϣ��ٰ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó���Һ��pH��
��3���Ʋ⡰��õ�pHС��7�������ɣ��ų�������ļ��룬ϡ������������Һ������pH��С��
�ʴ�Ϊ��
�ų���ϡ�Ͷ�ʹ��ҺpH��С�����أ�
����������
��4����ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ������ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t��
�������������Ũ�ȶ��ǵ�һ���2�������������ǵ�һ���4�����¶ȱ仯���ǵ�һ���4���������������������ǵڶ����2���������¶ȵĸı�Ҳ�ǵ������
 �����Ƴ�x=7��
�����Ƴ�x=7���ʴ�Ϊ��7��
��5���������ƺ����ᷢ���кͷ�Ӧ����Һ���¶Ȼ����ߣ�������ƿ�ڵ�ѹǿ����ʹU�ιܵ�Һ����ָ߶Ȳijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����U�ι������Һ���½���ӳ��Һ���Ȳ��ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
�ʴ�Ϊ��
U�ι������Һ���½���
��6��Ҫ��֤������Ƿ���ȷ����Ҫ�Ǽ���̼�������������ƵĴ��ڣ�һ�������ö�̼����ļ����Լ�������Һ�ļ�����ɣ���˼�����λ����Լ���̼��������ܼ����Ӧ�ļ��ֹ���ţ������̪���������Ƶļ��Խ��з����жϣ�
�ʴ�Ϊ��CaCl2��BaCl2��
��7�������������ݿ�֪����Һ�ڷ�Ӧǰ�����ʵ������仯�������Ƿ�Ӧ���ɵĶ�����̼�������ݴ˿����ݷ���ʽ���̼���Ƶ�������Ҳ��֪�������Ƶ�������
������̼�������ǣ�241.20g+10.00g-249.00g=2.2g
����Ʒ��̼���Ƶ�����Ϊx
H2SO4+Na2CO3=Na2SO4 +H2O+CO2��
106 44
x 2.2g
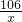 =
=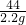
x=5.3 g
Na2CO3����������
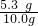 ��100%=53%
��100%=53%NaOH����������1-53%=47%
��8��������̼���ܶȽϴ����������ײд������Ķ�����̼�����²����Ķ�����̼������С��̼���Ƶ�������С��������������С��
�ʴ�Ϊ��
CO2���ܶȱȿ���������װ���У�������ղ�õ�����ƫ������ɵ�CO2����ƫС����Na2CO3����������ƫС��
����������һ����1��������֪����õ�pH��Сֱ��pHС��7��˵��������Һ�����ԣ�
��2����pH��ֽ�ⶨ��Һ��pHʱ��������ȷ�IJ���������
��3������ǿ������õ�pHС��7�������ɣ�����ϡ�����ϡ�͵����ã�
����������4���ɱ������ݣ����Ƴ�x��ֵ��
��5��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� U�ι������Һ���½��ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
��6����֤������Ƿ���ȷ����Ҫ�Ǽ���̼�������������ƵĴ��ڣ�һ�������ö�̼����ļ����Լ�������Һ�ļ�����ɣ�
��7���������ϵ�֪����Һ�ڷ�Ӧǰ�����ʵ������仯�������Ƿ�Ӧ���ɵĶ�����̼�������ݴ˿����ݷ���ʽ���̼���Ƶ�������Ҳ��֪�������Ƶ�������
��8�����ݶ�����̼���ܶȽϴ�������ɣ�
�����������Ķ����ϴ������кͷ�Ӧ�ĸ����Ӧ�ã�pH��ֽ�ⶨ��Һ��pHʱ�IJ�������������ʽ�����֪ʶ�����������������ߵ����ϵͣ��ڽ���ʱ����Ҫ������Ҫ�������⣬Ȼ��Ӧ�������յ�֪ʶ��һ������ɣ�

��ϰ��ϵ�д�
�����Ŀ
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮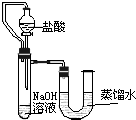
I��ʵ�鷽��
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨNaOH��ҺpHʱ����ȷ�IJ����ǣ� ��
��2������ǿ������õ�pHС��7�������ɣ� ��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������
NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10�˻�ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
��1������x= ��
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� �ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
II��ʵ���е��������
��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3��
��1��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м��������� ��Һ��������ɫ����������Ȼ�����ϲ���Һ�м����̪��Һ��������Һ�ʺ�ɫ����֤�˲��������ȷ�ģ�
��2��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ������ͼ��ʾ��ʵ�飮
ʵ�����ݼ�¼���£�
��ͨ�������������ݼ���������Ʒ�и��ɷݵ�����������д��������̣��� ��
��3����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ���� ��

I��ʵ�鷽��
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨNaOH��ҺpHʱ����ȷ�IJ����ǣ�
��2������ǿ������õ�pHС��7�������ɣ�
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������
NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10�˻�ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����
II��ʵ���е��������
��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3��
��1��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м���������
��2��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ������ͼ��ʾ��ʵ�飮

ʵ�����ݼ�¼���£�
| �� �� �� Ŀ | �� �� ʱ �� | ������g�� |
| ���� | 10.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 249.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 249.00 |
��3����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ����


 ����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮