��Ŀ����
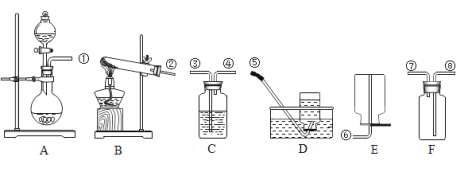
����Ŀ��ij�����ĩ�� Mg��MgO��Mg(OH)2 �е�һ�ֻ�����ɡ�ij��ѧС��Ϊ��̽���ù����ĩ�ijɷ֣������ͼ��ʾ��װ�ã��̶�װ��ʡ�ԣ���

����Ϣ���ϣ�
��1����ʵ�������£������ܶ�Ϊ 0.09g/L��
��2��Mg��MgO��Mg(OH)2 ���������ᷴӦ���ҷ�Ӧ����MgO + H2SO4 �T MgSO4 + H2O��Mg(OH)2 + H2SO4 �T MgSO4 + 2H2O
��3������þ��Һ�еμ�����������Һ�����������þ����MgSO4 + 2NaOH �T Na2SO4 + Mg(OH)2��
��ʵ����̣�
�����������װ�������ԣ�װ��ҩƷ������������
����������б Y �ιܣ�ʹ��Ʒ��ַ�Ӧ����������������� 55.6mL������ɸ���������������ԼΪ 0.005g����
������������װ�ã��� Y �ι��ڲ���Һ�м�������������Һ�����������ɳ����������ˡ�ϴ�Ӻ���Ƶó�������Ϊ 0.58g��
���ش����⣩
��1������װ�������Եļ���˵����ȷ���� ________��
A ��ˮ������һ�ξ��룬����γ�һ����Һ����˵��װ�ò�©��
B ��ˮ������һ�ξ��룬�������Һ����ƽ����˵��װ�ò�©����
��2������ˮԡ��ԭ����________��
��3����ʹ��Ʒ�������ַ�Ӧ����ȷ������________�����ţ���
A ������Һת�Ƶ���Ʒ�� B ��Ʒת�Ƶ�������Һ��
���ж���Ʒ��ȫ��Ӧ�������� ____________��
��4������ʵ����
��ͨ���������ƶϣ���Ʒ��һ�����е�������____________��ͨ���������������㣬��Ʒ��þԪ�ص�������Ϊ ____________�ˡ�
�����ۣ�����Ʒ�еijɷ���________��
��5���Ӹ�ʵ���еó���ȡ����þ�����ַ������ó���������Ӧ��Mg��MgO��Mg(OH)2 �ֱ������ᷴӦ�������ĵ�������Ũ�ȵ����ᣬ���ɵ�����þ����________����Ȼ�ͬ����
��6���û�ѧС��ͬѧ�������۵ó����½��ۣ���ȷ����________�����ţ���
A ���ø�ʵ�鷽�����ܼ������Ʒ�и����ʵ�����
B ʵ��ǰ��ͨ����Ʒ����������������Һ��������ȷ����Ʒ��ȫ��Ӧ
C �������������� Y �ι���ҩƷ��������������ɵ����������������ܼ������Ʒ�и����ʵ�����
���𰸡�A ��ȴ����ֹ�������������ڷ�Ӧ���ȶ����� A Y�ι��еĹ���ȫ����ʧ Mg 0.24g Mg��MgO ��� AB
��������
��1������װ�������Եļ�鷽���ǣ���ˮ������һ�ξ��룬ʹˮ�ܺ��������е�ˮ�γ�һ����Һ�����һ��ʱ���Һ���ֲ��䣬��˵��װ�ò�©������֮�����ʾװ��©�������A��
��2������ˮԡ��ԭ���ǣ���ֹ�������������ڷ�Ӧ���ȶ����ͣ��Ӷ���ֹ��������ȷ�������ȴ����ֹ�������������ڷ�Ӧ���ȶ����ͣ�
��3����Ϊ�˷�ֹ����ҩƷճ���������ϣ��ɽ�Һ̬��������Һת�Ƶ���Ʒ�У����A��
��þ������þ��������þ������ϡ���ᷴӦ�����Կ�������ҩƷ��ȫ��ʧ�ˣ���֤����Ʒ����ȫ��Ӧ�����Y�ι��еĹ���ȫ����ʧ��
��4����þ��ϡ���ᷴӦ��������þ��������������þ��������þ��ϡ���ᷴӦ��û���������ɣ���ʵ�������֪����Ʒ��һ������þ��
��������г������ɵķ���ʽΪMgSO4+2NaOH=Na2SO4+Mg��OH��2��
��Ʒ��þԪ�ص�������Ϊ��0.58g��![]() ��100%=0.24g��
��100%=0.24g��
���þ��0.24��
��������55.6mL������Ҫþ������Ϊx
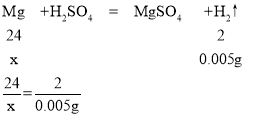
x=0.06g
��ʣ�����ȫ��Ϊ����þ����þԪ�ص�����Ϊ����0.36g-0.06g����![]() ��100%=0.18g
��100%=0.18g
����0.18g+0.06=0.24g�����Թ�����Ʒ����þ������þ��ɵġ����Mg��MgO��
��5��þ�����ᷴӦ�ķ���ʽΪMg+H2SO4=MgSO4+H2��������þ�����ᷴӦ�ķ���ʽΪ��MgO+H2SO4=MgSO4+H2O��������þ�����ᷴӦ��������þ��ˮ������ʽΪ��Mg��OH��2+H2SO4=MgSO4+2H2O�����������غ㶨�ɣ���������þ�е�������������������е���������ӣ��������ĵ�����Ũ�ȵ����ᣬ�������ɵ�����þ������ȣ������ȣ�
��6������ʵ���ṩ�����ݿ�֪�����ø�ʵ�鷽�����ܼ������Ʒ�и����ʵ�������ʵ��ǰ��ͨ����Ʒ������������ȫ��Ϊþ������������Һ��������ȷ����Ʒ��ȫ��Ӧ�����ǽ������������Y�ι���ҩƷ�������������������������ֻ�ܼ����������Ʒ����������þ�����������ܼ���������ɷֵ�������
��ѡ��AB��