��Ŀ����
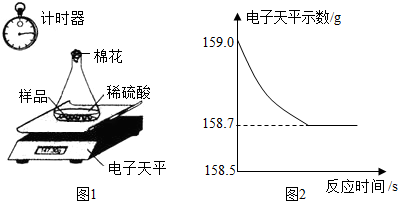
С��ͬѧ�ɼ���һЩ������Ʒ�������ʣ����ʲ�����ˮ������ϡ���ᷴӦ��������ͼ1��ʾװ�ý��з������ֱ�Ƶ���ƿ����������Ϊ44.1g��������Ʒ������Ϊ9.0g������ƿ�м�������ϡ�����������ʼ��¼������ƽ��ʾ������¼������ͼ2��
������������ݣ��ش��������⣺
��1��С�����ݴ�����ͼ������ȷ����
��2��������Ʒ������������������д�����̣�
��3�����㷴Ӧ��������Һ������������������������д�����̣�

������������ݣ��ش��������⣺
��1��С�����ݴ�����ͼ������ȷ����

��2��������Ʒ������������������д�����̣�
��3�����㷴Ӧ��������Һ������������������������д�����̣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
����������ϡ���ᷴӦ�������������������������������������Լ�������������������������������������һ�����Լ�����Ʒ���������������ͷ�Ӧ��������Һ����������������������
����⣺��1��A����Ӧ��������Ʒ�е�������Ȼ���ڣ���ѡ���ȷ��
B����������������Ϊ��159.0g-158.7g=0.3g����ѡ����ȷ��
C�����ŷ�Ӧ�Ľ��У���Һ���������ӣ���ѡ���ȷ��
���B��
��2������������Ϊx��������������������Ϊy��
Fe+H2SO4�TFeSO4+H2����
56 152 2
x y 0.3g
=
=
��
x=8.4g��y=22.8g��
��Ʒ��������������Ϊ��
��100%=93.3%��
����Ʒ��������������Ϊ93.3%��
��3��ϡ���������Ϊ��159.0g-44.1g-9.0g=105.9g��
�γ���Һ������Ϊ��8.4g+105.9g-0.3g=114g��
��Ӧ��������Һ��������������������Ϊ��
��100%=20%��
�𣺷�Ӧ��������Һ��������������������Ϊ20%��
B����������������Ϊ��159.0g-158.7g=0.3g����ѡ����ȷ��
C�����ŷ�Ӧ�Ľ��У���Һ���������ӣ���ѡ���ȷ��
���B��
��2������������Ϊx��������������������Ϊy��
Fe+H2SO4�TFeSO4+H2����
56 152 2
x y 0.3g
| 56 |
| x |
| 152 |
| y |
| 2 |
| 0.3g |
x=8.4g��y=22.8g��
��Ʒ��������������Ϊ��
| 8.4g |
| 9.0g |
����Ʒ��������������Ϊ93.3%��
��3��ϡ���������Ϊ��159.0g-44.1g-9.0g=105.9g��
�γ���Һ������Ϊ��8.4g+105.9g-0.3g=114g��
��Ӧ��������Һ��������������������Ϊ��
| 22.8g |
| 114g |
�𣺷�Ӧ��������Һ��������������������Ϊ20%��
������������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
���������������������ʵ�����࣬�����������ڴ�������ǣ�������
| A����Ȫˮ | B�������Ŀ��� |
| C����ȼ�� | D���ɱ� |
 С������ͼ����ʽ����ѧ֪ʶ���й��ɣ����мװ������ҡ���������������������������ʵľ�����࣬����д�������ݣ�
С������ͼ����ʽ����ѧ֪ʶ���й��ɣ����мװ������ҡ���������������������������ʵľ�����࣬����д�������ݣ�
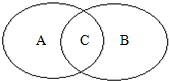 ��ͼ��ʾ�����A��B��Բ�ֱ��ʾ���Ϸ�Ӧ��������Ӧ�����������Բ�Ĺ�ϵ���ش��������⣮
��ͼ��ʾ�����A��B��Բ�ֱ��ʾ���Ϸ�Ӧ��������Ӧ�����������Բ�Ĺ�ϵ���ش��������⣮