��Ŀ����
����Ŀ��ѧϰ�����еĻ�ѧ�����������е�����ѧ�����������ߡ�
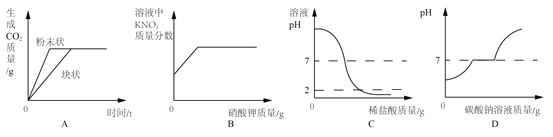
��1��С�ȷ������ӻ������˻��٣�Ϊ��ʹ�����翪������ζŨ����������ʣţ�̳�ϡ�������У������ʡ���������ͬ��������Ϊ�ʵ���________������ĸ��ţ���

A��CO(NH2)2 B��Ca(H2PO4)2 C��KNO3
��2����ɹ˪��Ҫ�ɷ��Ƕ������ѣ�TiO2���ȣ���Ҫ�������ɢ�������赲�����ߣ�����������ɹ����������������Ԫ�صĻ��ϼ�Ϊ____________��
��3����������ϴˢ��δ���ɣ�����һ��ʱ�����ֺ���ɫ��ߣ�����Ҫԭ��������________��ͬ���õĽ����д����ֹ�������������һ�ַ���_____________________��
��4����������ף���ϣ�СƼ���촵������������ԭ����__________________��
���𰸡� B +4 ������ˮ�� ϴ������ �����¶ȵ��Ż������
����������1������Ԫ�صĻ������ʣ���ѡB����2���������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㣬�������������Ԫ�صĻ��ϼ�Ϊx����x+��-2����2=0�����x=+4����3��������������������ˮ��ͬ���õĽ������������Ʒ�������ɷ�ֹ����Ʒ���⡣��4�����촵������������ԭ���ǽ����¶ȵ��Ż�����¡�

����Ŀ������ʵ���У���Ӧ�������Լ����۶���ȷ�������������( )
ѡ �� | A | B | C | D |
ʵ �� |
|
|
|
|
ʵ �� �� �� | ����ˮƿ��ʱ����ˮ ���Զ������ | ������ˮ������������ ���� | ����ȼ�������������� ���������������������ƶ�����ȴ�����º��� ����ͣ�ڿ̶���4������ | �������������ǵ�ľ�� ��ȼ���������������� ���ǵ�ľ��Ϩ�� |
ʵ �� �� �� | ˵��������ˮ�е��� �����ѹǿ������� ��С | ˵�����ʵ��ܽ����� �ܼ��������й� | ����Լռ��������� ���֮һ | �����е������Ⱥ��� ������������ |
A. A B. B C. C D. D
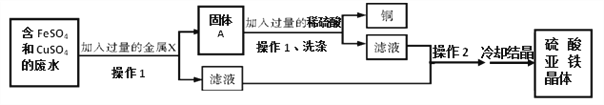
����Ŀ����ʵ�����У���ѧ��ȤС���ͬѧ������һƿ���ڷ��õ�Ca(OH)2��ĩ״�Լ������ǶԸ��Լ������˶���ʵ���������ȡ�Լ�19.8g������ƿ�У�����30.2g��ˮ�������������ƿ�����εμ�25g����ɷַ�Ӧ����ò���������ͼ�����£�������й���Ϣ�ش����⡣
��1�� | ��2�� | ��3�� | ��4�� | ��5�� | ��6�� | |
���������������g�� | 25 | 25 | 25 | 25 | 25 | 25 |
��ƿ�����ʵ�������g�� | 75 | 100 | a | 150 | 172.8 | 197.8 |
��1��a����ֵΪ____________��
��2��b����ֵΪ____________��
��3��������Լ����������Ƶ���������������������һλС����______________��

����Ŀ����ͼ��ijͬѧ�Զ�����̼����֪ʶ����������ͼ�����ַ�Ӧ�����Ͳ���������ʡ�ԣ���������Ҫ����գ�
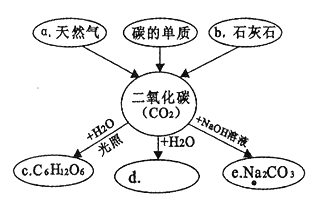
��1��д��aȼ�յĻ�ѧ����ʽ_________________________��
��2������d�Ļ�ѧʽ��_______________��
��3��������̼ת��ΪC����____�仯�����������ѧ������
��4�����������̼��Ϊ_________���������˹����ꡣ
��5��������̼�������������ЧӦ�����о�ָ�����õ�����ˮ����CO2���˹������漰����Ӧ2Mg2SiO4+2H2O+CO2�TMg3Si2O5(OH)4+ X ����������X Ϊ____________���ѧʽ����
��6����̼����ȼ�ջ���Ⱦ���������ƺͿ�������̼�������ڹ�ҵ�����������Դ��һֱ�ǿ�ѧ�Ҳ�и���Ŀ�ꡣ������NH3���ڿ����г��ȼ�յIJ����ǵ�����ˮ��ĿǰҺ�������dz�Ϊһ��DZ�ڵ����ȼ�ϡ�
Һ����Һ��ȼ������й����ݼ��±����ݴ��жϲ���ȷ����__________������ţ�
ȼ�� | �е㣨�棩 | ȼ�շ��ȣ�kJ/g�� | ��Է������� | ���庬����ը��Χ | Һ̬�ܶȣ�g/cm3�� |
���� | -252.6 | 119.20 | 2.0 | 4.0%��75.6% | 0.07 |
���� | -33.50 | 18.65 | 17.0 | 16%��25% | 0.61 |
A����й©�������б�������������ը B��������Ϊȼ��ʱ�����Լ�������ЧӦ
C������Һ�����������ö� D���������İ�����ֱ�ȼ�գ���ų���������