��Ŀ����
��ѧ������������أ���������ѧ��ѧ֪ʶ�ش��������⣺
�ż�ͥʹ�õ�ȼ�ϣ�����ǹ�װҺ��ʯ����������Ҫ�ɷ��б��飨��ѧʽΪC3H8����
�ٱ����ڿ����г��ȼ�պ��������ֳ���������仯ѧ����ʽΪ�� ���ù����ǰѻ�ѧ��ת��Ϊ �ܡ�
�����극��ͨ���ǰ�ȼ����ع�����Ϩ����������Ϩ���ԭ���� �����ţ���
A���������� B���Ƴ���ȼ�� C�������¶ȵ���ȼ���Ż������
�����˵�ʹ�����Ӧ��ʱ����ˮ�ֲ�Ϳ��һ��ʳ���ͣ��������ɷ����ԭ����
��
���п��ڼ䣬Ϊ��С���������棬����Ϊ������������ͣ����������㡢�����⡢��������
�ٴӡ�����Ӫ�����ĽǶȷ����������С��ʳ����Ӧ���ӵ�Ӫ����Ϊ ��
��Ϊ��С����ʳ��ȫ������ʳƷ�в���ʳ�õ��� ��A.���д����������Ƶ���� B.�ü�ȩ���ݵİײ� C.��С�մ����Ƶ���ͷ
�ż�ͥʹ�õ�ȼ�ϣ�����ǹ�װҺ��ʯ����������Ҫ�ɷ��б��飨��ѧʽΪC3H8����
�ٱ����ڿ����г��ȼ�պ��������ֳ���������仯ѧ����ʽΪ�� ���ù����ǰѻ�ѧ��ת��Ϊ �ܡ�
�����극��ͨ���ǰ�ȼ����ع�����Ϩ����������Ϩ���ԭ���� �����ţ���
A���������� B���Ƴ���ȼ�� C�������¶ȵ���ȼ���Ż������
�����˵�ʹ�����Ӧ��ʱ����ˮ�ֲ�Ϳ��һ��ʳ���ͣ��������ɷ����ԭ����
��
���п��ڼ䣬Ϊ��С���������棬����Ϊ������������ͣ����������㡢�����⡢��������
�ٴӡ�����Ӫ�����ĽǶȷ����������С��ʳ����Ӧ���ӵ�Ӫ����Ϊ ��
��Ϊ��С����ʳ��ȫ������ʳƷ�в���ʳ�õ��� ��A.���д����������Ƶ���� B.�ü�ȩ���ݵİײ� C.��С�մ����Ƶ���ͷ
�Ţ�C3H8 + 5O2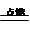 3CO2 + 4H2O ���ܻ����
3CO2 + 4H2O ���ܻ����
����һ��3�֣��ڶ���1�֣���4�֣�
��B ��2�� ��
��ʹ�˵���ˮ�Ϳ������������� ��2�֣�
�Ǣ�ά���� ��A B
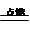 3CO2 + 4H2O ���ܻ����
3CO2 + 4H2O ���ܻ��������һ��3�֣��ڶ���1�֣���4�֣�
��B ��2�� ��
��ʹ�˵���ˮ�Ϳ������������� ��2�֣�
�Ǣ�ά���� ��A B
�ű���ȼ��������ˮ�Ͷ�����̼���ڸ�������ԭ����֪�����극��ͨ���ǰ�ȼ����ع����ǿ�ȼ���3��������Ҫ�������࣬����������Ҫ���е����ʣ���������Ҫ���е����ʺ���֬������������Ҫ���е����ʡ�ˮ���������Σ�����ȱ��ά���أ���ͨ��ʳ���߲˺�ˮ�������䣬���˹���ʳ�ú��������Ƶ�ʳƷ���°�����ȩ���ж����к����壬����ʳ�ã���ȩ��ʹ�����ʵĽṹ���ƻ��������������к���Ҳ��ı���Ҫ�������ʵ�ʳƷ��Ʒ��

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ