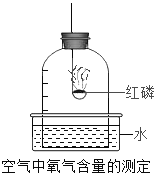
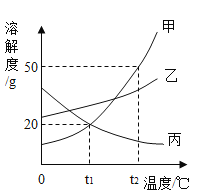
��Ŀ����
����Ŀ��Ϊ�ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ѧϰС���ȡ��6g��Ʒ�����ʽ����Ȼ��ƣ������ձ��в�����ϡ���ᡣ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g���������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
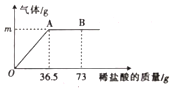
��1��A��ʱ���������������mΪ______g��
��2����Ʒ��̼���Ƶ�������������ȷ��0.1%��д��������̣���_____��
��3��B��ʱ���ձ�����Һ�к��е���������______��д���ӷ��ţ���
���𰸡�2.2 88.3% Na+��H+
��������
���������������������ϡ�����������ϵͼ���ṩ�˽����������Ϣ��
��1�����������غ㶨�ɿɵã����ɵĶ�����̼������Ϊ��6g+36.5g-40.3g=2.2g
��2���⣺����Ʒ��̼���Ƶ���������Ϊx��
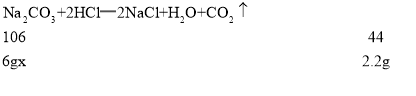
![]()
���x=88.3%
����Ʒ��̼���Ƶ���������Ϊ88.3%��
��3������A��λǡ����ȫ��Ӧ������B��Ϊϡ�������������B��ʱ���ձ�����Һ������Ϊ�Ȼ��ƺ�ʣ���HCl�����Ժ��е��������ǣ�Na+��H+��

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ