��Ŀ����
ij��ѧ��ȤС���ͬѧ��������װ�ý���ʵ������ȡ�����̽�����������̽�����ش��������⣺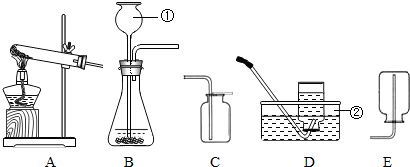
��1��д����������������ƣ���
��2��ʵ�����ø��������ȡ������Ӧѡ�õ�װ�������
��3��ʵ����ͨ����ϡ�����ʯ��ʯ��Ӧ��ȡCO2���÷�Ӧ�Ļ�ѧ����ʽΪ
��4��ʵ�����ڳ������Ƶõ���Ȳ��C2H2�������г�����������������CO2��H2S�������±߷����в���������װ�ã���������ֱ�߱�ʾ��������ע��Ҫ���Լ���
��������1������ʵ���ҳ�����������ʶ������⣻
��2���ø��������ȡ������Ӧѡ�õ�װ����Ҫ���ȣ�����������ˮ���Ժ��ܶȣ�ѡ���ռ�������
��3����ϡ�����ʯ��ʯ��Ӧ��ȡCO2��װ�ò���Ҫ���ȣ�������̼������ˮ����������ˮ����ֻ���������ſ�������
��4��������������������CO2��H2S����Ҫ���ǣ������ܳ������̣ܶ���Ҫͨ������Һ��
��2���ø��������ȡ������Ӧѡ�õ�װ����Ҫ���ȣ�����������ˮ���Ժ��ܶȣ�ѡ���ռ�������
��3����ϡ�����ʯ��ʯ��Ӧ��ȡCO2��װ�ò���Ҫ���ȣ�������̼������ˮ����������ˮ����ֻ���������ſ�������
��4��������������������CO2��H2S����Ҫ���ǣ������ܳ������̣ܶ���Ҫͨ������Һ��
����⣺��1������ʵ���ҳ�����������ʶ������⣮��Ϊ���ٳ���©����ˮ�ۣ�
��2���ø��������ȡ������Ӧѡ�õ�װ����Ҫ���ȣ�����������ˮ���Ժ��ܶȣ�ѡ���ռ�������������ˮ���������ſ�������
��3����ϡ�����ʯ��ʯ��Ӧ��ȡCO2��װ�ò���Ҫ���ȣ�������̼������ˮ����������ˮ����ֻ���������ſ�������
��4��������������������CO2��H2S����Ҫ���ǣ������ܳ������̣ܶ���Ҫͨ������Һ������������Һ����
�ʴ�Ϊ����1���ٳ���©����ˮ�ۣ�2��AD��AC��2KMnO4
K2MnO4+MnO2+O2������3��CaCO3+2HCl�TCaCl2+H2O+CO2����BC
��4��Ҫ��1�������ܳ������̣ܶ�Ҫ��2������Һ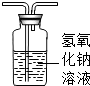
��2���ø��������ȡ������Ӧѡ�õ�װ����Ҫ���ȣ�����������ˮ���Ժ��ܶȣ�ѡ���ռ�������������ˮ���������ſ�������
��3����ϡ�����ʯ��ʯ��Ӧ��ȡCO2��װ�ò���Ҫ���ȣ�������̼������ˮ����������ˮ����ֻ���������ſ�������
��4��������������������CO2��H2S����Ҫ���ǣ������ܳ������̣ܶ���Ҫͨ������Һ������������Һ����
�ʴ�Ϊ����1���ٳ���©����ˮ�ۣ�2��AD��AC��2KMnO4
| ||
��4��Ҫ��1�������ܳ������̣ܶ�Ҫ��2������Һ

������ѧ��Ƚ��ø�����غ��ù���������Һ�Ͷ���������ȡ����������������ȡ�����Ͷ�����̼ԭ�����Ʒ������������ע��㣮

��ϰ��ϵ�д�
�����Ŀ
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����


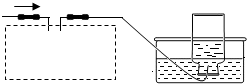
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������