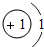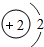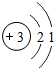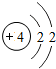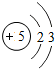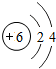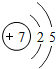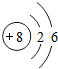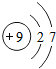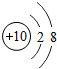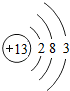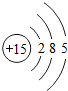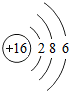��Ŀ����
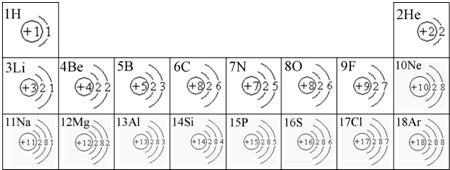
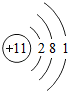
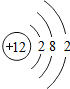
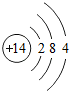
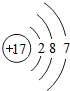
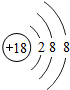
��ͼ��ԭ������ǰ18��Ԫ�ص�ԭ�ӽṹʾ��ͼ�����ڱ������ж��ɵ�ͼʾ��
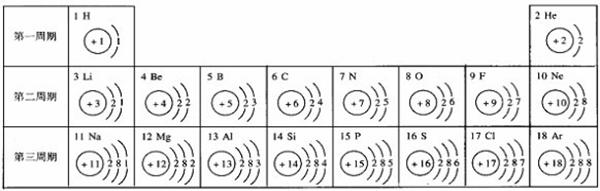 ��1��9��Ԫ��F���� ��ѡ��������ǽ�������Ԫ�أ��γɼ����ӵ����ӷ���_ _��
��1��9��Ԫ��F���� ��ѡ��������ǽ�������Ԫ�أ��γɼ����ӵ����ӷ���_ _��
��2��д��12��Ԫ����17��Ԫ���γɻ�����Ļ�ѧʽ��______________��
��3������ͼ���ҿ���̽�������¹��ɣ����������= _______=ԭ������=__________��
��4���о��������ڶ����ڴ�3��~9��Ԫ�ص�ԭ�ӵ��Ӳ�����ͬ���˵���������˶Ժ�����ӵ�����������ԭ�ӵİ뾶��С���ҷ����������ڴ�11��~17��Ԫ��ԭ�Ӱ뾶�仯�����ǣ�______ ________��
��5����ͼ���ҵó��ˡ�ͬһ����Ԫ�ص�ԭ�ӵ��Ӳ�����ͬ���˵���������Ĺ��ɣ����⣬�һ�̽������һЩ���ɣ�______ ________��
��1���ǽ���Ԫ�ء�F-�� ��2��MgCl2
��3��ԭ������=������=�˵����=���������
��4��ԭ�ӵ��Ӳ�����ͬ���˵����������������ԭ�Ӱ뾶Ҳ����С��
��5��ͬһ�壬������������ͬ���������ɣ�

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
�����Ŀ
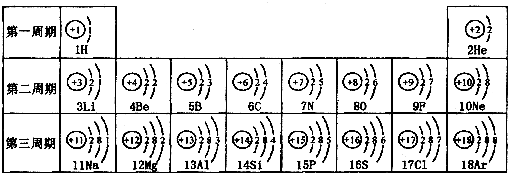
��ͼ��ԭ������ǰ18��Ԫ�ص�ԭ�ӽṹʾ��ͼ�����ڱ������ж��ɵ�ͼʾ��
| ��һ���� | 1H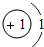 | 2He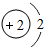 | ||||||
| �ڶ����� | 3Li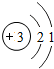 | 4Be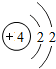 | 5B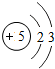 | 6C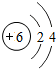 | 7N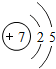 | 8O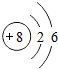 | 9F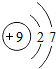 | 10Ne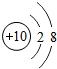 |
| �������� | 11Na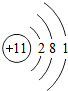 | 12Mg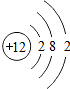 | 13Al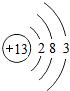 | 14Si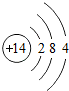 | 15P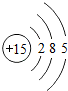 | 16S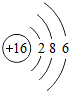 | 17Cl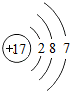 | 18Ar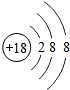 |
��2��д��12��Ԫ����17��Ԫ���γɻ�����Ļ�ѧʽ��________��
��3������ͼ���ҿ���̽�������¹��ɣ����������=________=ԭ������=________��
��4���о��������ڶ����ڴ�3�š�9��Ԫ�ص�ԭ�ӵ��Ӳ�����ͬ���˵���������˶Ժ�����ӵ�����������ԭ�ӵİ뾶��С���ҷ����������ڴ�11�š�17��Ԫ��ԭ�Ӱ뾶�仯�����ǣ�________��
��5����ͼ���ҵó��ˡ�ͬһ����Ԫ�ص�ԭ�ӵ��Ӳ�����ͬ���˵���������Ĺ��ɣ����⣬�һ�̽������һЩ���ɣ�________��