��Ŀ����
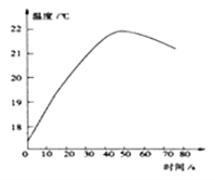
����Ŀ��ʵ�����м�����ƿ���õ��������ƹ��壬ijѧϰС��Ϊ���о���������������������ʵ��: (���ӳ�ʾ����λΪ��)
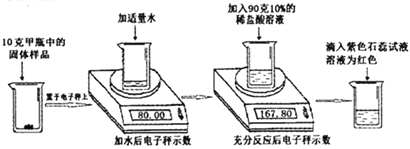
(1)������ɫʯ����Һ����ҺΪ��ɫ��˵����Ӧ����Һ��____�ԡ�
(2)����ʵ���в����Ķ�����̼��������Ϊ______�ˡ�
(3)�����ƿ������Ʒ��̼���Ƶ���������____________��
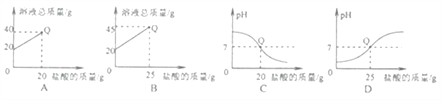
(4)ijͬѧ��ȡ10����ƿ�еĹ�����Ʒ,��100��15%��ϡ���ᰴͬ����������ʵ�飬����Ϊ���ܹ�����Ʒ���ʳ̶���Σ�ϡ������������Ҫʹ��ʯ����Һ�������˵�����������жϵ�ԭ��__________��
���𰸡� �� 2.2 53% ��100g15%��ϡ�������ʵ�飬ϡ����һ����������ϡ����������Ҫ��ʯ����Һ��
�����������⿼���˸��ݻ�ѧ����ʽ���м��㡣���������غ�����õ�������̼�����������ݶ�����̼��������ϻ�ѧ����ʽ������Ʒ��̼���Ƶ���������һ�����������Ʒ��̼���Ƶ�����������
��1����ɫʯ����Һ��������Һ���ɫ��������ɫʯ����Һ����ҺΪ��ɫ��˵����Ӧ����Һ�����ԡ�
��2�����������غ㣬�����Ķ�����̼��������Ϊ80.00g+90g-167.80g=2.2g��
��3���������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
![]() x=5.3g
x=5.3g
������Ʒ��̼���Ƶ���������=![]() ��100%=53%��
��100%=53%��
��4������Ʒ��ȫ���ʣ���Ʒȫ�����̼���ơ�
�裺��10g̼���Ʒ�Ӧ�����������Ϊy��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98
10g y
![]() y=9.25g������ϡ���������=
y=9.25g������ϡ���������=![]() =61.64g��61.64g<100g��ϡ���������
=61.64g��61.64g<100g��ϡ���������
����Ʒû�б��ʣ���Ʒ����10g���������ơ�
�裺��10g�������Ʒ�Ӧ�����������Ϊz��
2NaOH+H2SO4=Na2SO4+2H2O
80 98
10g z
![]() z=12.25g������ϡ���������=
z=12.25g������ϡ���������=![]() =81.67g��81.67g<100g��ϡ���������
=81.67g��81.67g<100g��ϡ���������
���Բ��ܹ�����Ʒ���ʳ̶���Σ�����100g15%ϡ�����ϡ����һ�����������������жϵ�ԭ������100g15%��ϡ�������ʵ�飬ϡ����һ����������ϡ����������Ҫ��ʯ����Һ��

����Ŀ��������ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ��������ƾ��������˺��棬����ɷֽ���̽��������ش��������⡣
��������
a������ƾ����þƾ����Ȼ��ƺ��������ư�һ���������Ȼ���Ƴɡ�
b���Ȼ��ơ��Ȼ���Һ�������ԡ�
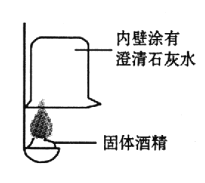
��������⣩
�پƾ����Ƿ���̼Ԫ�أ�
�ڹ���ƾ��е����������Ƿ���ʣ�
��ʵ��̽����
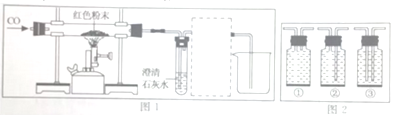
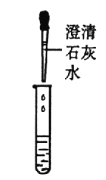
�ٰ���ͼ��ʾ����ʵ�飬�����ձ��ڱ���һ���Ĥ���ɵó��ƾ��к���̼Ԫ�صĽ��ۣ�������_______________��
��ȡ��������ƾ����ձ��У���������ˮ����ܽ���ã������ձ��ײ��а�ɫ���������û�ѧ����ʽ��ʾ�ó���������γɵģ�_______________�ɴ�˵�����������ѱ��ʡ�
��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ��������̽����
����ͬѧȡ�ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ����______________ |
ʵ����� | ��Һ������������ | ��Һ����̼���� |
����ͬѧ��Ϊ����ʵ�鲻��֤����Һ��һ�����������ƣ�������_______________
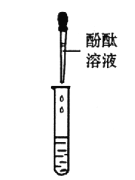
������ȡ�ձ����ϲ���Һ���������Ȼ�����Һ����ַ�Ӧ���ã�ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼����������ʵ���м������Ȼ�����Һ��Ŀ����_____________
��ʵ����ۣ�С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�