��Ŀ����
����Ŀ��ij�о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɡ��ڻ�ѧ��ʦ�İ����£�ѡ��98%��Ũ���ᡢ��ͭ��Ʒ��������ʵ�鲢���㡣

ʵ�������150g 9.8%��ϡ���ᡣ
��1�����㣺��ҪŨ����Լ8.2mL����Ҫˮ�����Ϊ_________mL��ȡ����ֵ��
��2����ȡŨ�����ˮ������Ͳ��ȡ8.2 mLŨ�����һ�������ˮ�������ȡ98%��Ũ����ʱ���Ӷ������ᵼ��������Һ������������_________9.8%������ڡ�����С�ڡ����ڡ�����
��3��_________ ����4��װƿ����ǩ��
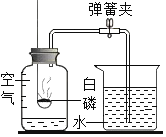
ʵ�������ͼ1��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ɰֽ��ĥ����Ŀ���ǣ�_________��
��2��ʵ����������У��ټ�¼������D��Һ��λ�ã��ڼ�������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�á��۴�B�в���������������ָ������º�¼������D��Һ��λ�ã�����A��B�μ������Լ����ݽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��������еIJ�����˳����_________������ţ�����¼������D��Һ��λ��ʱ��������ƽ���⣬��Ӧ_________��
��3��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________��
��4����ʵ���У�пͭ�Ͻ���ϡ�����ַ�Ӧ��ò������������ΪV L��Ϊ����Ͻ���п��ͭ��������������Ҫ�õ����������е�_________��
A�������ϡ������������� B����Ӧ��B��ʣ����������
C��ʵ��ǰ��Ӧװ���п�������� D��ʵ���������������ܶ�
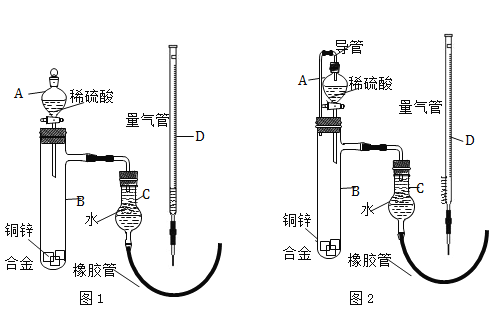
��5��С��ͬѧ������������������õ�п������������ƫ���ֽ�װ�ø�Ϊ��ͼ2��ʾ���Ľ���Ŀ����_________��
���𰸡�135 С�� ���� ��ȥ���������Ĥ �ڢ٢ܢۢ� ���������ܵ�Һ��ʹC��Dװ������Һ����ƽ Zn +H2SO4 = ZnSO4+H2�� BD �����˼���ϡ���������Բ�õ����������Ӱ�죬ʹʵ������ȷ
��������
ʵ�����(1)���㣺����ҪŨ���������Ϊx��x��98%=150g��9.8%��ã�x=15g����Ҫˮ�����Ϊ150g-15g=135g�����Ӷ�����ʹ��ȡ��Ũ�������ƫС���ᵼ��������Һ������������С��9.8%��(3)���ȣ�ϡ��ʱһ��ҪǿŨ��������������ע��ˮ������Ͻ�����(4) ��4��װƿ����ǩ������Һ��9.8%��������Һ����ǩΪ�� ��ʵ�����(1) ��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ɰֽ��ĥ����Ŀ���dz�ȥ�Ͻ���������Ĥ��(2)Ҫ�����ſ�Һ���������ⶨ����������������������������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ������¼������D��Һ��λ������A��B�μ������Լ�����B�в���������������ָ������º�¼������D��Һ��λ�ã���B��ʣ�������ˣ�ϴ�ӣ�������أ�����˳��Ϊ���ڢ٢ܢۢ�����¼������D��Һ��λ��ʱ��������ƽ���⣬��Ӧʹ���������ܵ�Һ��ʹC��Dװ������Һ����ƽ��(3)����п�����ᷴӦ����������ͭ�����������������õ��������ϡ������������Ӧ�Ļ�ѧ����ʽΪ�� Zn+H2SO4�TZnSO4+H2����(4) ����Ͻ���п��ͭ��������������Ҫ�õ���Ӧ��B��ʣ������������ʵ���������������ܶ���(5) �Ľ���Ŀ���������˼���ϡ���������Բ�õ����������Ӱ�죬ʹʵ������ȷ��
��ʵ�����(1) ��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ɰֽ��ĥ����Ŀ���dz�ȥ�Ͻ���������Ĥ��(2)Ҫ�����ſ�Һ���������ⶨ����������������������������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ������¼������D��Һ��λ������A��B�μ������Լ�����B�в���������������ָ������º�¼������D��Һ��λ�ã���B��ʣ�������ˣ�ϴ�ӣ�������أ�����˳��Ϊ���ڢ٢ܢۢ�����¼������D��Һ��λ��ʱ��������ƽ���⣬��Ӧʹ���������ܵ�Һ��ʹC��Dװ������Һ����ƽ��(3)����п�����ᷴӦ����������ͭ�����������������õ��������ϡ������������Ӧ�Ļ�ѧ����ʽΪ�� Zn+H2SO4�TZnSO4+H2����(4) ����Ͻ���п��ͭ��������������Ҫ�õ���Ӧ��B��ʣ������������ʵ���������������ܶ���(5) �Ľ���Ŀ���������˼���ϡ���������Բ�õ����������Ӱ�죬ʹʵ������ȷ��

 ��ս100��Ԫ����Ծ�ϵ�д�
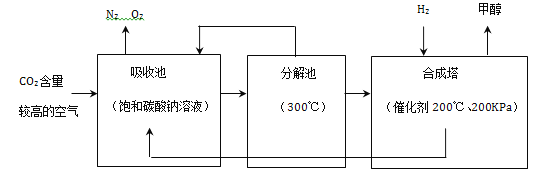
��ս100��Ԫ����Ծ�ϵ�д�����Ŀ����ʵġ�̼�������˵����������һ���߽���̼�������硣
��1�������±��ṩ����Ϣ����д�йغ�̼���ʵĶ�Ӧ���ԡ�
������; | ���ʯ�и�� | ʯī���缫 | ����̿��ˮ |
��Ӧ���� | ��________ | ��________ | ��_______ |
��2��Һ̬������̼������������˾ȵ����ҷ����Ļ��֣�����˵����ȷ����_________��
A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3������Ķ�����̼�Ӿ��ˡ�����ЧӦ����д��һ����������ЧӦ��ǿ�ŷŵĽ���_________��
��4��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú��_____����Ȼ�������Ƕ�����_______�������������������������Դ��д����Ȼ���м�����ȫȼ�յĻ�ѧ����ʽ��________��