��Ŀ����
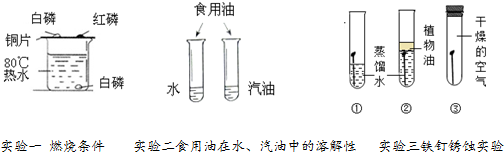
2��ͼ1��Сǿ���α����е�һ����ѧʵ�飬ʵ��ʱ���Ƕ��ŵ�һ�����ŵĴ̼�����ζ����������ԭʵ�������������ƣ���ͼ2��ʵ��װ������ͼ��
ʵ�����
����ʢ��20mL����ˮ���ձ��е���3-4����ɫ��̪��Һ��������ȣ��۲���Һ��ɫ��
��ȡ����������Һ���Թ��У������������μ�Ũ��ˮ���۲�����
����B��C�Թ��ڷֱ���5mL�ձ��еķ�̪��Һ���ձ���ʣ��ķ�̪��Һ����ʵ�������Ƚϣ���Ȼ���ڿ��ձ��е�����������ˮ���ã�����A��D�Թ��зֱ���2mLŨ��ˮ�������ô���Ƥ���ĵ��ܰ�ʵ��ͼ2���Ӻã�����D�Թܷ�������ˮ�У��۲켸���ӣ�
��ش�
��1���ӷ��ӵĽǶȷ������ŵĴ̼�����ζ������ԭ���Ƿ����Dz����˶��ģ�
��2�����в����ٵ�ʵ��Ŀ�������Աȣ�
��3�������۹۲쵽��������B�з�̪��Һ������죬C�з�̪��Һ���ٱ�죨��C�з�̪��Һ��B���ȱ�죩��
��4���ɴ˿��Եõ���ʵ������� �ٷ����ڲ�ͣ���˶����¶�Խ�ߣ������˶�Խ�죻
��5���Ľ���ʵ����ŵ��ǣ���һ�㼴�ɣ����ٶԻ�������Ⱦ�������Աȣ�̽����Ӱ������˶����ʵ����أ���
���� ��1�����ݷ��ӵ����ʽ��н��
��2������B�ձ��з��з�̪��Һ��A�ձ��ڵ��������Աȷ�����
��3������ʵ���Ŀ�ĺ��������������Ŀ�ģ�
��4�����ݷ����ڲ�ͬ�¶����˶�������������
��5������Ŀ�е�ͼ�ο�֪���Ľ���ʵ����ŵ㣺�ܾ�����ֹ�����ݳ���Ⱦ�����ȣ�
��� �⣺��1�������Dz����˶��ģ����Ի��ŵ����ŵĴ̼�����ζ������
��2��B��A��C�ձ��������γɶԱȣ�˵����ˮ��ʹ��̪��Һ��죬����ˮ����ʹ��̪��ɫ��
��3���¶�Խ�ߣ������˶�Խ���ң�B�Թ��ڳ����½��У���C�Թ�����ˮ�н��У���C�Թ��еķ�̪��Һ�ܿ���ɫ��B�Թ��еķ�̪��Һ�������ɫ��
��4���¶�Խ�ߣ������˶�Խ���ң�B�Թ��ڳ����½��У���C�Թ�����ˮ�н��У���C�Թ��еķ�̪��Һ�ܿ���ɫ��B�Թ��еķ�̪��Һ�������ɫ����̪��ɫ˵�������������˶������˷�̪��Һ��C�Թܺܿ��죬B�Թܻ�����죬˵���¶�Խ�ߣ������˶��ٶ�Խ�죻
��5���Ľ����ʵ���ŵ��ǰ����ӷ��������е����٣���������Ⱦ���������Ҹ�ʵ���ܹ�ͨ��ʵ��Ա�̽�����ӵ��˶����¶��йأ�
�ʴ�Ϊ����1�������Dz����˶��ģ�
��2�����Աȣ�
��3��B�з�̪��Һ������죬C�з�̪��Һ���ٱ�죨��C�з�̪��Һ��B���ȱ�죩��
��4���ٷ����ڲ�ͣ���˶������¶�Խ�ߣ������˶�Խ�죻
��5�����ٶԻ�������Ⱦ�������Աȣ�̽����Ӱ������˶����ʵ����أ���
���� ���⿼���˷��ӵĴ������˶�������Ӱ�����أ���ƶԱ������Ͽ��Ʊ��������������ǽ���Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 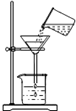 ���� | B�� | 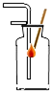 ���� | C�� | 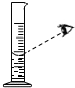 ��ȡҺ����� | D�� | 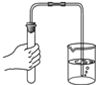 ��������� |
| A�� | 3O2--��ʾ������ԭ�� | |
| B�� | SO3--��ʾһ��������������к���������Ԫ�� | |
| C�� | 2Al3+--��ʾ���������Ӵ�������λ������� | |
| D�� | $\stackrel{-3}{Al}$N--��ʾ�������У���Ԫ����-3�� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | һ����̼����ұ������--һ����̼�ܹ�ȼ�� | |
| B�� | �ɱ������˹�����--�ɱ�������ͬʱ���մ������� | |
| C�� | ������ʳƷ������--�����Ļ�ѧ���ʲ����� | |
| D�� | ͭ�������쵼��--ͭ�������õĵ����� |
| A�� | ͨ��ʹˮ�ֽ� | B�� | ���ֽ����������Һ | ||
| C�� | ����������ȷֽ� | D�� | ��������ȷֽ� |
 �˵�θҺ�ﺬ�����������ᣬ�����������������θ����ڹ��࣬�˻�е�θʹ��С��������һ�ֿ���ҩ������θ������ҩ�����ҩ��˵����IJ���������ͼ��ʾ��
�˵�θҺ�ﺬ�����������ᣬ�����������������θ����ڹ��࣬�˻�е�θʹ��С��������һ�ֿ���ҩ������θ������ҩ�����ҩ��˵����IJ���������ͼ��ʾ��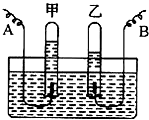 ijͬѧ�������ˮʵ��ʱ����������ͼ��ʾ�ļ���װ�ã��ش��������⣺
ijͬѧ�������ˮʵ��ʱ����������ͼ��ʾ�ļ���װ�ã��ش��������⣺