��Ŀ����
����Ŀ��ˮ�����⡢������Ԫ����ɵĻ������һ�����ǿ�ѧ�����ڴ���ʵ�յĻ����ϵó��ġ�
�����ϣ�(1)��ʯ�ҵ���Ҫ�ɷ�����������(NaOH)��������(CaO)������ˮ�����������̼��Ӧ��
(2)ͬ��ͬѹ�£����������ȵ��ڷ������ȡ�
(1)�о�������ȼ��ʵ����������ʶˮ��ɵĿ�ʼ��
�������ڵ�ȼǰһ��Ҫ_____��
������ͬ�����£���ʹ1L������ȫȼ�գ������������ԼΪ_____L��
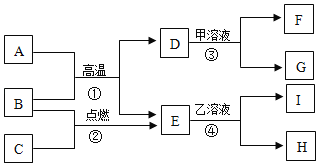
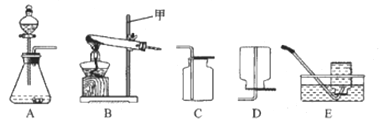
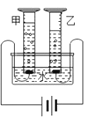
(2)��ѧ������������ⷨ��֤����ˮ�����(��ͼ)���÷�Ӧ�Ļ�ѧ����ʽΪ_____________�����ʱ��ˮ�����������ϡ���������������Һ����������___________��ͨ��һ��ʱ��� ��Ͳ�����ռ�����������____________(����������)�������ּס�������Ͳ���ռ��������������С�� 1��2����ԭ�������___________________(д��һ������)��
(3)������������һʵ��֤����ˮ����ɡ�����ˮ����ͨ��һ���պ������ǹ�ܣ�����õ���������ͬʱǹ�ܱ����к�ɫ����(���������Ҫ�ɷ�)���ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
(4)����������ԭ����ͭ��ʵ��Ҳ����֤��ˮ�����(��ͼ)��
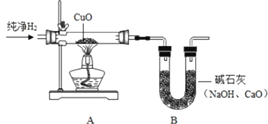
����ͼ����ʵ��װ�á����װ�õ������ԡ�װҩƷ���̶�ʵ��װ�ú���Ҫʵ�鲽���У� a��ͨ��������b����ȼ�ƾ��ƣ�c��Ϩ��ƾ��ƣ�d��ֹͣͨ�����������У���ͨ�������ȼ�ƾ��Ƶ�Ŀ����__��
��Ӳ�ʴֲ������з�����������_________����Ӧ�Ļ�ѧ����ʽ��_________��
��װ�� A �еIJ����ܺ����������ڷ�Ӧǰ���������Ϊ m1��װ�� B �����������ڷ�Ӧǰ���������Ϊm2���ݴ˿ɼ����ˮ�� H��O Ԫ�ص�������Ϊ_____________(�ú� m1��m2 �Ĵ���ʽ��ʾ)��ʵ��ʱ���ñ�ֵ�����Դ���1��8��ԭ�������_____(�����)��
A�������к���ˮ����
B��װ��A �����ɵ�ͭ�ֲ��ֱ�����
C�������е�ˮ������������̼�Ƚ���װ�� B ��
D��������ˮ����Һ��������Ӳ�ʲ�������
���𰸡��鴿 2.5L(��2.38L) 2H2O![]() 2H2����O2�� ��ǿˮ�ĵ����� ���� ������ˮ�е��ܽ�������������(�缫�벿������������Ӧ) 3Fe+4H2O
2H2����O2�� ��ǿˮ�ĵ����� ���� ������ˮ�е��ܽ�������������(�缫�벿������������Ӧ) 3Fe+4H2O![]() Fe3O4+4H2 �ž���������ֹ��ը �����ɺ�ɫ��Ϊ��ɫ H2��CuO
Fe3O4+4H2 �ž���������ֹ��ը �����ɺ�ɫ��Ϊ��ɫ H2��CuO![]() Cu��H2O ��m2-m1����m1 ABCD
Cu��H2O ��m2-m1����m1 ABCD
��������
(1)���������п�ȼ�ԣ�������ȼ���ܻᷢ����ը�����������ڵ�ȼǰһ��Ҫ�鴿��
���ɷ���ʽ��2H2+O2![]() 2H2O��֪1L������ȫȼ������0.5L��������Ϊ����Լռ���������
2H2O��֪1L������ȫȼ������0.5L��������Ϊ����Լռ���������![]() ��21%�����Կ��������2.5L��2.38L��
��21%�����Կ��������2.5L��2.38L��
(2)���ˮ������������������Ӧ�Ļ�ѧ����ʽ�ǣ�2H2O![]() 2H2����O2�������ʱ��ˮ�������������ϡ���������������Һ������������ǿ�����ԣ�ͨ��һ��ʱ����ּס�������Ͳ���ռ��������������С��1��2���ɴ˿��ж����ҹ�������һ���ǵ�Դ�ĸ��������е������������������ԭ���������ͬ�����£�������������������ˮ��
2H2����O2�������ʱ��ˮ�������������ϡ���������������Һ������������ǿ�����ԣ�ͨ��һ��ʱ����ּס�������Ͳ���ռ��������������С��1��2���ɴ˿��ж����ҹ�������һ���ǵ�Դ�ĸ��������е������������������ԭ���������ͬ�����£�������������������ˮ��
(3)�������֪����Ӧ��������ˮ������������������������������Ӧ�������Ǹ��£����Ի�ѧ����ʽΪ��3Fe+4H2O![]() Fe3O4+4H2��
Fe3O4+4H2��
(4)��ʵ��տ�ʼ��ͨ������Ŀ���ǣ��ž���������ֹ�����������Ļ������ȼ������ը��
������������ͭ�ڼ��ȵ�����������ͭ��ˮ������Ӳ�ʲ������г��ֵ�����Ϊ��ɫ��ĩ��ɺ�ɫ����ѧ����ʽΪ��H2��CuO![]() Cu��H2O��
Cu��H2O��
��װ��A�еIJ����ܺ����������ڷ�Ӧǰ���������Ϊm1��������ͭ�м��ٵ���Ԫ�ص�������Ҳ����ˮ����Ԫ������Ϊm1��װ��B�����������ڷ�Ӧǰ���������Ϊm2��������������ͭ��Ӧ���ɵ�ˮ����������ˮ�������ǣ�m2������ˮ����Ԫ������Ϊ��m2-m1������H��OԪ�ص�������Ϊ��m2-m1����m1�������к���ˮ������װ��A �����ɵ�ͭ�ֲ��ֱ����������ڿ����е�ˮ�Ͷ�����̼�����Bװ�á�������ˮ����Һ��������Ӳ�ʲ������У������½������1��8��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�