��Ŀ����
���ݡ�ʳƷ��ȫ������ع涨��ʳƷ������ͭ������ʳƷ��ҵ�üӹ������������������Ǧ�����Լӹ�����Ƥ������ij��ʳƷ�ӹ�����Ҫʹ��100g������������Ϊ8%������ͭ��Һ����ͨ������ش�
��1������Һ������������ g������ȡ10g��Һ������Һ����������Ϊ ��
��2��ʵ����������ͭ���壨����ʽΪCuSO4?5H2O������100��8%������ͭ��Һ�����ȡ����ͭ���� g������֪��Է���������CuSO4.5H2OΪ250��CuSO4Ϊ160��
��1������Һ������������
��2��ʵ����������ͭ���壨����ʽΪCuSO4?5H2O������100��8%������ͭ��Һ�����ȡ����ͭ����
���㣺�й��������������ļ���
ר�⣺��Һ����ɼ��������������ļ���
��������1��������������=��Һ���������ʵ��������������з������
��2������ͭ���壨����ʽΪCuSO4?5H2O������ˮ������������ͭ���ݴ˽��з������
��2������ͭ���壨����ʽΪCuSO4?5H2O������ˮ������������ͭ���ݴ˽��з������
����⣺��1��ij��ʳƷ�ӹ�����Ҫʹ��100g������������Ϊ8%������ͭ��Һ������Һ������������100g��8%=8g��
��Һ���о�һ�ԣ�����ȡ10g��Һ������Һ������������Ϊ8%��
��2������ͭ���壨����ʽΪCuSO4?5H2O������ˮ������������ͭ������100��8%������ͭ��Һ����Ҫ��ȡ����ͭ����Ϊ8g�£�
��100%��=12.5g��
�ʴ�Ϊ����1��8��8%����2��12.5��
��Һ���о�һ�ԣ�����ȡ10g��Һ������Һ������������Ϊ8%��
��2������ͭ���壨����ʽΪCuSO4?5H2O������ˮ������������ͭ������100��8%������ͭ��Һ����Ҫ��ȡ����ͭ����Ϊ8g�£�
| 160 |
| 250 |
�ʴ�Ϊ����1��8��8%����2��12.5��
�����������ѶȲ����������������������йؼ��㲢�������������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
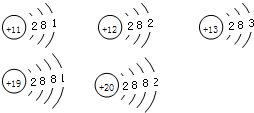
���з������ܱ�ʾһ��ԭ�ӣ���ʾһ��Ԫ�أ����ɱ�ʾ�������ʵ��ǣ�������
| A��H |
| B��C60 |
| C��Cu |
| D��O2 |
2007���ҹ��������ˮ�ա��Ϳ�չ���й�ˮ�ܡ���������������Ϊ��ˮ����չ���г��ᡱ�������й�ˮ��֪ʶ�У�������ǣ�������
| A��ˮ��һ��������ܼ�����Ȼ���е�ˮ���������� |
| B��ˮ��Ca2+��Mg2+ �����������ˮ��ĸ�Ӫ���� |
| C������л�����ķ����ܽ���ˮ��Ӳ�� |
| D��������ˮ�������ŷ� |
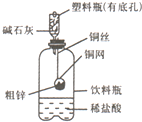 ij��ѧ��ȤС��������ͼװ�ö�ij��п��Ʒ���д��ȼ�⣮����д����ʵ�鱨�森
ij��ѧ��ȤС��������ͼװ�ö�ij��п��Ʒ���д��ȼ�⣮����д����ʵ�鱨�森