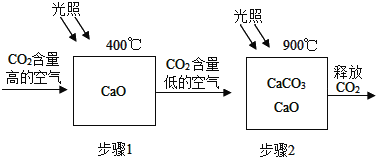
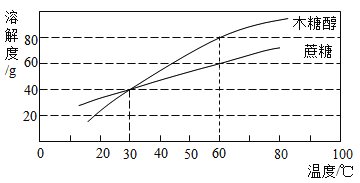
��Ŀ����
����Ŀ���������ƣ�Na2FeO4����һ�ָ�Ч���ˮ������,��ҵ�ϳ����ô������ƣ�NaClO����������������Ӧԭ���û�ѧ����ʽ��ʾΪ3NaClO+2Fe��NO3��3+10NaOH=2Na2FeO4��+3NaCl+6NaNO3+5H2O����NaClO����Է�������Ϊ74.5��Na2FeO4����Է�������Ϊ166����
��1����Fe��NO3��3�У���Ԫ�غ͵�Ԫ�ص�������Ϊ______�������ȣ���
��2���ֳ�ȡ44.7g��������,������Ƶø������Ƶ������Ƕ���?����ʽ���㣩______
���𰸡�4:3 66.4g
��������
��1����Fe��NO3��3�У���Ԫ�غ͵�Ԫ�ص�������Ϊ��56����14��3��=4��3��
��2���裺�������Ƶ�����Ϊx��
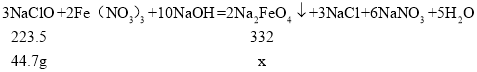
![]() x=66.4g��
x=66.4g��
�����ɵõ�������ص�����Ϊ66.4g��

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ