��Ŀ����
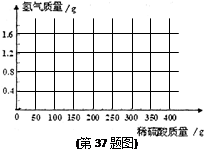
��2010���㽭����36������ѧ�ҷ��֣�����ȼ���ֲ��ķ�ΧԼռ�����������10�����䴢���� ú��ʯ�ͺ���Ȼ���ܺ͵�������������Ϊֹ���ֵĺ������ֵ�Ŀ��
��Դ����ȼ��Ҳ��Ϊ������ˮ��������û������ǿ��ɿ�ȼ�����뷽��֮һ������CO2ע�˺��ļ���ˮ���ﴢ�㣬CO2�ϼ�������γ�ˮ���������ܽ�����ˮ�����еļ�����ӡ����ߡ����Ӷ����䡰�û�������(ԭ����CH4��nH2O+CO2==CO2��nH2O+CH4)��
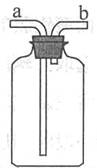
(1)ͨ������£�CH4��һ����ɫ����ζ��������ˮ���ܶȱȿ���С�����塣����ͼ��ʾ��
װ���ռ�CH4����ʱ����Ӧ������� (ѡ�a����b��)��ͨ�롣
(2)�����������ܹ�ʵ�֣���ôҪ��ȡ8�ֵ�CH4���壬��������CO2���ٶ�?
��Դ����ȼ��Ҳ��Ϊ������ˮ��������û������ǿ��ɿ�ȼ�����뷽��֮һ������CO2ע�˺��ļ���ˮ���ﴢ�㣬CO2�ϼ�������γ�ˮ���������ܽ�����ˮ�����еļ�����ӡ����ߡ����Ӷ����䡰�û�������(ԭ����CH4��nH2O+CO2==CO2��nH2O+CH4)��

(1)ͨ������£�CH4��һ����ɫ����ζ��������ˮ���ܶȱȿ���С�����塣����ͼ��ʾ��
װ���ռ�CH4����ʱ����Ӧ������� (ѡ�a����b��)��ͨ�롣
(2)�����������ܹ�ʵ�֣���ôҪ��ȡ8�ֵ�CH4���壬��������CO2���ٶ�?
(1)b(2��)
(2)����������ҪCO2������Ϊx����
x=22��(2��)
����������ҪCO2��22�֡�
(2)����������ҪCO2������Ϊx����
x=22��(2��)
����������ҪCO2��22�֡�
���⿼����dz���������ռ����������ݻ�ѧ����ʽ���㡣
(1)ϴ��ƿ��ʹ��ʱ�����ռ��ܶȱȿ����ܶȴ������ʱ�������dz����̳������ռ��ܶȱȿ����ܶ�С������ʱ�������Ƕ̽�������������ˮ�����ռ�����ʱ��һ���Ƕ̽���������ΪCH4���ܶȱȿ���С�����������Ƕ̽�������b��
(2) ����������֪�����δ֪����Ȼ����ݻ�ѧ����ʽCH4��nH2O+CO2==CO2��nH2O+CH4�б���ʽ��⡣
����������ҪCO2������Ϊx��
CH4��nH2O+CO2==CO2��nH2O+CH4
44 16
X 8t
44:16=x:8t x=22t
����������ҪCO222�֡�
(1)ϴ��ƿ��ʹ��ʱ�����ռ��ܶȱȿ����ܶȴ������ʱ�������dz����̳������ռ��ܶȱȿ����ܶ�С������ʱ�������Ƕ̽�������������ˮ�����ռ�����ʱ��һ���Ƕ̽���������ΪCH4���ܶȱȿ���С�����������Ƕ̽�������b��
(2) ����������֪�����δ֪����Ȼ����ݻ�ѧ����ʽCH4��nH2O+CO2==CO2��nH2O+CH4�б���ʽ��⡣
����������ҪCO2������Ϊx��
CH4��nH2O+CO2==CO2��nH2O+CH4
44 16
X 8t
44:16=x:8t x=22t
����������ҪCO222�֡�

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ