��Ŀ����
27��������������ϡ���ᷴӦ��ʵ��ʱ�۲쵽�����ݲ�������Һ����ɫ���dz��ɫ��
��1��д����Ӧ�Ļ�ѧ����ʽ
��2����Ӧ����Һ����Ҫ����Cl-��
��3���������е�֪����Һ����ɫͨ��������Һ�е����Ӿ����ģ��ɴ�����Ϊ�÷�Ӧ����Һ��dz��ɫ����
��1��д����Ӧ�Ļ�ѧ����ʽ
Fe+2HCl=H2��+FeCl2
����2����Ӧ����Һ����Ҫ����Cl-��
Fe2+
��H2O
�������ӷ��ţ�����3���������е�֪����Һ����ɫͨ��������Һ�е����Ӿ����ģ��ɴ�����Ϊ�÷�Ӧ����Һ��dz��ɫ����
Fe2+
�������ӷ��ţ����ֳ����ģ���֤��ʵ�鷽������ʵ����Ӧǰ��������Һ����ɫ�ģ�������п�ŵ��Ȼ�������Һ�У���Һ��dz��ɫ��Ϊ��ɫ������������ҺΪdz��ɫ��������ҺΪ��ɫ�ȣ������������𰸾��ɣ�
����������1��������������Ļ�ѧ��Ӧԭ�����ɣ�
��2��������Һ����ɷ������ɣ�
��3��Fe2+����ɫ���ٸ�����Һ�������ɫ�������ɣ�
��2��������Һ����ɷ������ɣ�
��3��Fe2+����ɫ���ٸ�����Һ�������ɫ�������ɣ�
����⣺��1�����������ᷴӦ�ų��������ʴ�Ϊ��Fe+2HCl=H2��+FeCl2��
��2���������ᷴӦ�����Ȼ�������Һ���Ȼ�������ˮ�л�����Cl-��Fe2+���ܼ�Ϊˮ��H2O����
�ʴ�Ϊ��Fe2+��H2O��
��3����Ϊϡ������ɫ������H+��Cl-��ɫ����Ϊ�Ȼ�������Һ����ɫ�������������У�Cl-��Fe2+����Cl-��ɫ������Fe2+����ɫ��
�ʴ�Ϊ��Fe2+����Ӧǰ��������Һ����ɫ�ģ�
��2���������ᷴӦ�����Ȼ�������Һ���Ȼ�������ˮ�л�����Cl-��Fe2+���ܼ�Ϊˮ��H2O����
�ʴ�Ϊ��Fe2+��H2O��
��3����Ϊϡ������ɫ������H+��Cl-��ɫ����Ϊ�Ȼ�������Һ����ɫ�������������У�Cl-��Fe2+����Cl-��ɫ������Fe2+����ɫ��
�ʴ�Ϊ��Fe2+����Ӧǰ��������Һ����ɫ�ģ�
��������Щ����������ɫ�ģ���Fe2+����ɫ��Fe3+�Ի�ɫ��Cu2+����ɫ��ͬѧ����Ҫע����Щ���ӵ�������ɫ��

��ϰ��ϵ�д�
�����Ŀ
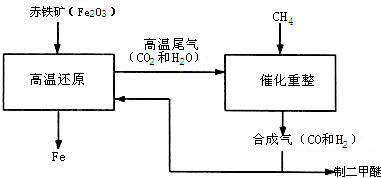
 CO+H2 ��CO+H2O==CO2+H2
CO+H2 ��CO+H2O==CO2+H2






