��Ŀ����
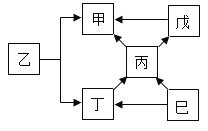
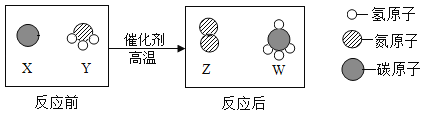
����Ŀ��A��B��C��D��E��FΪ������ˮ��������̼���������ơ�̼���ơ�̼������������е�һ�֣�����ͼ��ʾ��ת����ϵ��
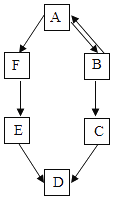
��1��A�ɹ����������仯ѧʽΪ________��
��2��C�׳���ʯ�ң�Bת����C�Ļ�ѧ����ʽΪ________��
��3��D�Ļ�ѧʽΪ________��
��4��Fת��ΪE�Ļ�ѧ����ʽΪ________��
��5��ͼ���������ʼ�����ת����ϵ����AB������ͼ���ҳ�������ת����ϵ�����ü�ͷ��ȫ____________��
���𰸡�O2 CaO+H2O=Ca(OH)2 CaCO3 CO2+2NaOH=Na2CO3+H2O 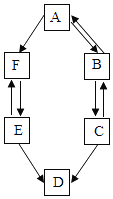
��������
�������⣺A��B��C��D��E��FΪ������ˮ��������̼���������ơ�̼���ơ�̼������������е�һ�֣�����A�ɹ������������A��������C�׳���ʯ�ң����C���������ƣ�������B�����ת������B�ܹ�ת��Ϊ�������ƣ����B��ˮ�������ܹ�ת��ΪF�����F�Ƕ�����̼��F�ܹ�ת��ΪE��E���������ƶ���ת��ΪD�����DӦ��Ϊ̼��ƣ�EΪ̼���ƣ�������֤����ת����ϵ��������ȷ��
�ɷ�����֪��
��1��A����������ѧʽΪ��O2��
��2��C���������ƣ�ˮ�������Ʒ�Ӧ�����������ƣ���ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
��3��DΪ̼��ƣ���ѧʽΪ��CaCO3��
��4��������̼���������Ʒ�Ӧ����̼���ƺ�ˮ����ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��
��5��������̼��̼���ƿ����ת����ˮ���������ƿ����ת������ͼ��
 ��
��

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�����Ŀ��Ϊ�˼��ijʯ��ʯ��Ʒ��̼��Ƶĺ������ס��ҡ���������λͬѧ������������ͬ����������Ʒ��ַ�Ӧ������ʵ��ⶨ����Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ������þ����±���
��ͬѧ | ��ͬѧ | ��ͬѧ | ��ͬѧ | |
��ȡʯ��ʯ��Ʒ������g�� | 12.5 | 12.5 | 12.5 | 12.5 |
���������������g�� | 30.0 | 40.0 | 55.0 | 70.0 |
ʣ������������g�� | 6.5 | 4.5 | 2.5 | 2.5 |
�Իش�
����Ʒ��̼��Ƶ���������Ϊ���٣���
��12.5g��Ʒ������ϡ���ᷴӦ��ɲ���������̼���ٿˣ���д��������̣�
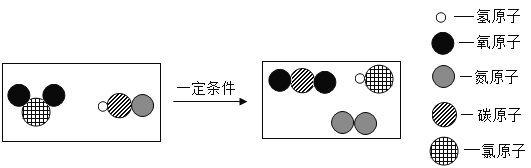
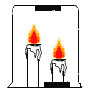
����Ŀ��ij��ѧ��ȤС����ʵ����������̽��ʵ�飬��ͼ��ʾ�����ǽ�һ֧�ձ�����![]() ֧ȼ�ŵĸߵͲ�ͬ�������ϣ��������Ǹߵ�������������������һͬ̽����
֧ȼ�ŵĸߵͲ�ͬ�������ϣ��������Ǹߵ�������������������һͬ̽����

��������⣩Ϊʲô�ߵ���������
��������裩����1���ߵ���������Ϊ_____ ����2���ߵ���������Ϊ�ϲ������������ġ�
��ʵ����֤1��Ϊ����֤����1�Ƿ���ȷ��С��ͬѧ����������ʵ�顣
���� | ���� | ���� |
�����г���ʯ��ˮ����ֽ�ֱ�����ձ��Ķ����͵ײ�������ֽ�ϵμӷ�̪����ɫ�����ձ���ס��ȼ�ĸߵ����۲�ߵ���ֽ��ɫ˳����ͼ�� | _____. | �ձ��ϲ������̼Ũ�Ƚϸߡ���������Ϩ���������̼Ũ�ȹ����йء��漰���Ļ�ѧ����ʽ��_____. |
С��ȴ��С���Ľ��۱�ʾ���ɣ���������װ���ռ�һƿ80%������̼��![]() �����Ļ�����壬���ұ����������ƽ�_____��С�콫ȼ�ŵ����������ռ��Ļ�������У�����_____���Ӷ�֤��С���Ľ��۲���ȷ��
�����Ļ�����壬���ұ����������ƽ�_____��С�콫ȼ�ŵ����������ռ��Ļ�������У�����_____���Ӷ�֤��С���Ľ��۲���ȷ��
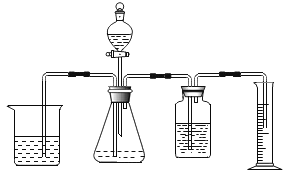
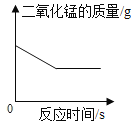
��ʵ����֤2��Ϊ����֤����2��С�������ֳּ������飬��������������̼���������ձ���������������֧����ӵ�ȼ��ȫ��Ϩ����������Ͷ�����̼��Ũ�ȱ仯����ͼ��ʾ��ʣ������Ũ��![]() ��������̼Ũ��
��������̼Ũ��![]() ��������̼Ũ�����࣬������Ũ�������½���
��������̼Ũ�����࣬������Ũ�������½���
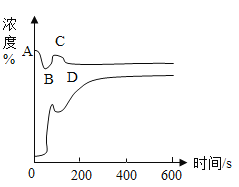
��ʵ����ۣ�_____.
����˼���ۣ�
���ӷ֣�����ȷ�ش�����С�⣬�����![]() �ֵĽ���������ѧ�Ծ��ֲܷ�����
�ֵĽ���������ѧ�Ծ��ֲܷ�����![]() �֡�
�֡�
����ͼ�����ߣ�װ��������Ũ�ȣ��仯��������_____���![]() ������ʱ����ȫ��Ϩ�����������
������ʱ����ȫ��Ϩ�����������![]() �Ρ�
�Ρ�![]() �Ρ�
�Ρ�![]() �α仯ԭ��
�α仯ԭ��
![]() �Σ�_____.
�Σ�_____.
![]() �Σ�_____.
�Σ�_____.
![]() �Σ�_____.
�Σ�_____.