��Ŀ����
����Ŀ��ͨ��һ��Ļ�ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɣ���������ʦ�ṩ��һЩʵ��װ�á�������ͼ�ش����⣺
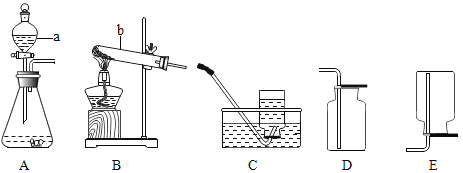
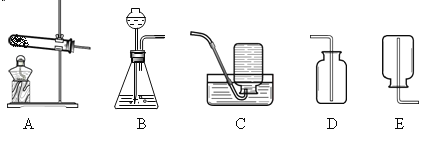
��1��д��ͼ�б�ŵ��������ƣ�a______b______��
��2��д��ʵ������Aװ����ȡ�����Ļ�ѧ����ʽ______��
��3��ͨ���������ϵ�֪���ٰ���NH3��һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���ڰ����ڼ�����������������ͭ��Ӧ����ͭ��ˮ�Ϳ����к����������塣С��ͬѧ�����Ȼ�狀��������ƵĹ���������ȡ��������Ӧѡ��� ��ȡװ������� _______��������ĸ��ţ�
��4����д������������ͭ��Ӧ�Ļ�ѧ����ʽ______��
���𰸡���Һ©�� �Թ� 2H2O2![]() 2H2O+O2�� BE 2NH3��3CuO
2H2O+O2�� BE 2NH3��3CuO![]() 3Cu��3H2O��N2
3Cu��3H2O��N2
��������
��1��д��ͼ�б�ŵ��������ƣ�a�ֽ�©��b�Թܣ�
��2��ʵ�����ù�Һ����װ��A��ȡ������ֻ���ù���������Һ�Ͷ������̹���������������Ӧԭ��Ϊ��2H2O2![]() 2H2O+O2����
2H2O+O2����
��3��С��ͬѧ�����Ȼ�狀��������ƵĹ���������ȡ��������Ӧѡ��������װ��B��
����NH3��һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���������ſ������ռ�������ѡ��װ��E��
��4�������ڼ�����������������ͭ��Ӧ����ͭ��ˮ�͵���������������ͭ��Ӧ�Ļ�ѧ����ʽ��2NH3��3CuO![]() 3Cu��3H2O��N2��
3Cu��3H2O��N2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����������Ļ������ڹ�ũҵ�������������й㷺��Ӧ�á�
��һ����ҵ������
��1����ҵ���ÿ�����ȡ��������������Ҫ��ֵķе����±���
��� | ���� | ���� |
�е㣨�棩 | -196 | -183 |
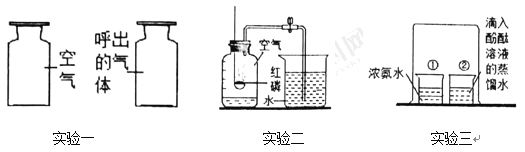
���������͵�����________��ͬ����Һ̬��������õ�������
��2���ҿ�ʢ��Һ̬�����ı���ƿ�ǣ�����ȼ�ŵ�ľ������ƿ���Ϸ����ɹ۲쵽__________������ĸ����
A ľ��Ϩ��
B ��ȼ�ո���
C ľ����Ϩ�𣬺�ȼ�ո���
���������������
��3��ij��ͥ����������ʪ��ƿװ�á�������ʱ�۲쵽ƿ�������ݡ�ʪ��ƿװ����������ͼ�����ڸ�װ�ã�����˵����ȷ����____________������ĸ����

A b��������������
B b���������������������Ϲ�
C ʹ�ø�װ�ÿ��Թ۲��Ƿ����������
D ʹ�ø�װ�ÿ��Թ۲��������������
��4�����������к��������ӵ���_______�����ţ���
��CO2 �ں�ˮ ��H2O2 �ܿ��� ��Һ�� �������
��������ҵ������
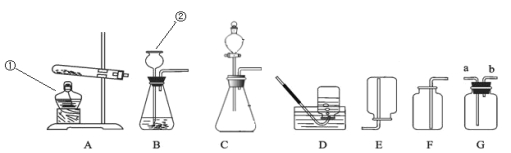
��5���������ʱ���ù������ƣ�CaO2��������ˮ�к����������뵽�������ƣ�CaO2���Ĵ����ã�С����ʵ��ʱ��������в��벢����ͼװ�ý���ʵ�飺
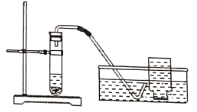
�����룩CaO2��H2O��Ӧ����ʵ������ȡ������
��ʵ����̣�
��____________����һʵ�鲽�裩��
�����Թ��м���CaO2��H2O���м���ϸС���ݻ����ų������������뼯��ƿ�У������ռ��������塣���õ��ڶ��죬����ƿ��ֻ�ռ����������壬���Թܺ����м���ϸС���ݻ����ų���
��ʵ����ۣ�CaO2��H2O��Ӧ__________����ܡ����ܡ�������ʵ������ȡ������
�������뷴˼��
�ٽ������ʵ�飬С����Ϊ�������ʱ��CaO2�����������ŵ���_______________��
��С�����ô���CaO2��ע���������______________________��
��ͬѧ�Ƕ�CaO2��H2O��Ӧ��������������£���.�������ƺ����� ��.̼��ƺ�����
����Ϊ����_________������ţ�һ���Ǵ���ģ�������_____________________��
���ղ����д��CaO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________��
���ģ�CaO2Ʒ�ʵ�����
С����������������CaO2��Ʒһ�������ʲ�����Ԫ�أ����ð���ǩ��ͼ��ʾ���Լ��㣺

��6��CaO2�и�Ԫ������Ԫ�ص�������Ϊ____________��________gCaO2��24.5g KClO3�к���Ԫ�ص������൱����һ����Ʒ���ٺ�CaO2________g��
��7����Ϊ���ԭ����������̼ԭ������Ϊ2.0��1026kg��ij����ԭ�ӵ����ԭ������Ϊ18�������ԭ������Ϊ___________��1026kg��
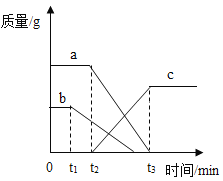
����Ŀ������ʯ��ʯ��Դ�ḻ��ij����С��ͬѧΪ�˲ⶨʯ��ʯ��̼��Ƶ�����������ȡij��ʯ��ʯ��Ʒ10.0g���ձ��У���100gϡ�����5�μ����ձ��У���ַ�Ӧ��(���ʲ���ϡ���ᷴӦ)�����ʣ������������¼���£�
���� | 1 | 2 | 3 | 4 | 5 |
����ϡ��������/g | 20 | 20 | 20 | 20 | 20 |
ʣ���������/g | 8.0 | 6.0 | 4.0 | 2.5 | X |
����㣺(1)X��ֵΪ__________��
(2)��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ_____________
(3)����ϡ�������ʵ���������Ϊ________��(д���������)