��Ŀ����
������ˮ��������̼������������Ҫ�����ʣ���1�������������ܹ����������� �������������� ��
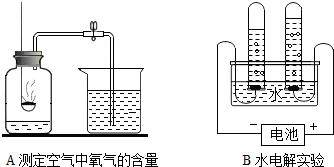
��2��A��B�����о�������ɵ�ʵ�飮
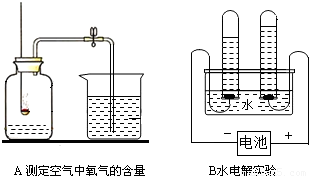
Bʵ���и����õ��������� ��Aʵ���з�����Ӧ�Ļ�ѧ����ʽΪ��ȼ�ճ���ҩƷΪ���ף� ��
��3��������̼���к���Ҫ�����ã��������к���������������ЧӦ��Ϊ���������ŷţ���ѧ���������о�������Ķ�����̼�������ڴ����ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ��飨CH4�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4�����д�ʩ�У������ڽ��ʹ����ж�����̼�������� ��
A�����ӻ�ʯȼ�ϵ�ʹ��
B��ֲ�����֣�����ֲ�����
C����������̼���Ϊ����ѭ�����ã�
���𰸡���������1���������ʾ�����;������������;�ж�����Ӧ�����ʣ�
��2������ʵ�������ʵ������жϣ�����ɷ�Ӧ��ѧ����ʽ����д��
��3�����ݷ�Ӧ��������Ϣ����ɷ�Ӧ�Ļ�ѧ����ʽ��д��
��4�����������Զ�����̼��Ӱ�죮
����⣺��1���������������ԣ���ά�ֺ�����������̼��֧��ȼ�ղ���ȼ�տ��������ˮ���������Ƚ�����Χ�¶ȣ����������
�ʴ�Ϊ��������ˮ��������̼��
��2����������϶࣬Ϊ��������ȼ���������������ף�
�ʴ�Ϊ��������4P+5O2 2P2O5��
2P2O5��
��3��������̼�������ڴ����ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ���CH4��
��ѡCO2+4H2 CH4+2H2O��
CH4+2H2O��
��4��A�����ӻ�ʯȼ�ϵ�ʹ�ã���ʯȼ��ȼ�����ɶ�����̼�����Ӷ�����̼������
B��ֲ�����֣�����ֲ�������������ÿɰѶ�����̼����ת��Ϊ���������Ķ�����̼��
C����������̼���Ϊ����ѭ�����ã��������ö�����̼�����Ͷ�����̼������
��ѡBC��
������ˮ���ʱ������������������������������������������������2����
��2������ʵ�������ʵ������жϣ�����ɷ�Ӧ��ѧ����ʽ����д��
��3�����ݷ�Ӧ��������Ϣ����ɷ�Ӧ�Ļ�ѧ����ʽ��д��
��4�����������Զ�����̼��Ӱ�죮
����⣺��1���������������ԣ���ά�ֺ�����������̼��֧��ȼ�ղ���ȼ�տ��������ˮ���������Ƚ�����Χ�¶ȣ����������
�ʴ�Ϊ��������ˮ��������̼��
��2����������϶࣬Ϊ��������ȼ���������������ף�
�ʴ�Ϊ��������4P+5O2
 2P2O5��
2P2O5����3��������̼�������ڴ����ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ���CH4��
��ѡCO2+4H2
 CH4+2H2O��
CH4+2H2O����4��A�����ӻ�ʯȼ�ϵ�ʹ�ã���ʯȼ��ȼ�����ɶ�����̼�����Ӷ�����̼������
B��ֲ�����֣�����ֲ�������������ÿɰѶ�����̼����ת��Ϊ���������Ķ�����̼��
C����������̼���Ϊ����ѭ�����ã��������ö�����̼�����Ͷ�����̼������
��ѡBC��
������ˮ���ʱ������������������������������������������������2����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
������ˮ��������̼������������Ҫ�Ļ�ѧ���ʡ�
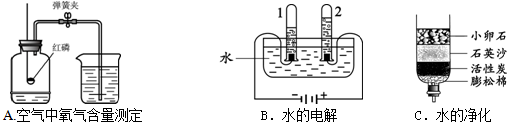
��1�����������������������ԭ���� ��
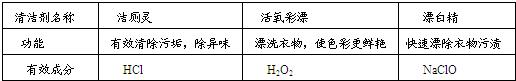
��2��ˮ��һ�ֺܺõ��ܼ������м��ּ�������������ˮ���ܼ����ƶ��ɵģ��书�ܼ���Ч�ɷ����±���ʾ��
| �������� | ����� | ������Ư | Ư�� |
| ���� | ��Ч����۹�������ζ | Ưϴ���ʹɫ�ʸ����� | ����Ư���������� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
�� NaClO����Ԫ�صĻ��ϼ�Ϊ ��
�� ��ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������� ��
�� ������顱�롰Ư�������ܻ��á�����������ײ����ж���������ͬʱ���Ȼ��ƺ�ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������̼���������Ϊ�µ�ʱ������ȫ����̼�����ָ�����к����ٵ��������Ӷ�����̼�ر��Ƕ�����̼���ŷš��������ʽ���ϡ���̼��������е� ����������ţ���
�ٽ����������Ķ�����̼��ʯ��ˮ����
���ٿ�˽�ҳ��������������ͨ���߳���
�۹㷺ʹ��һ���Կ��ӡ�һ�������ϴ�
���õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô��漰��ӡ��