��Ŀ����
������ˮ��������̼������������Ҫ�����ʣ���1��A��B�����о�������ɵ�ʵ�飮
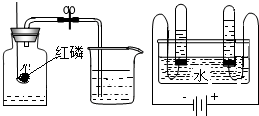
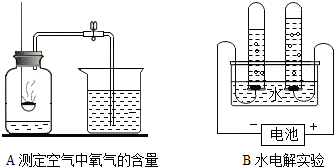
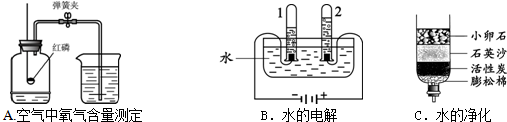
A���ⶨ�����������ĺ��� B�����ˮʵ��
Aʵ���з�����Ӧ�Ļ�ѧ����ʽ��
Bʵ���и����õ���������
��2��������̼���к���Ҫ�����ã��������к���������������ЧӦ��Ϊ���������ŷţ���ѧ���������о�������Ķ�����̼�������ڴ���
�ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ��飨CH4�����÷�Ӧ�Ļ�ѧ����ʽΪ
��3�����д�ʩ�У������ڽ��ʹ����ж�����̼��������
A�����ӻ�ʯȼ�ϵ�ʹ�� B��ֲ�����֣�����ֲ����� C����CO2���Ϊ����ѭ��ʹ�ã�
��������1��A������д��ѧ����ʽ�IJ��裻д��ע�ȣ�д����Ӧ����ʽ��B���ݣ����������⡱��ʵ����۽��н��
��2���������е���Ϣ������д��ѧ����ʽ�IJ��裻д��ע�ȣ�д����Ӧ����ʽ��
��3�����ݷ�ֹ����ЧӦ�Ĵ�ʩ��ֲ�����֣���ֲ���ݣ������̻��������������ú̿��ʯ�ͺ���Ȼ����ʹ�ã���������Դ�����н��
��2���������е���Ϣ������д��ѧ����ʽ�IJ��裻д��ע�ȣ�д����Ӧ����ʽ��
��3�����ݷ�ֹ����ЧӦ�Ĵ�ʩ��ֲ�����֣���ֲ���ݣ������̻��������������ú̿��ʯ�ͺ���Ȼ����ʹ�ã���������Դ�����н��
����⣺��1��A������д��ѧ����ʽ�IJ��裻д��ע�ȣ�Aʵ���з�����Ӧ�Ļ�ѧ����ʽΪ��4P+5O2
2P2O5��
B���ݣ����������⡱��ʵ����ۣ���֪Bʵ���и����õ�������Ϊ��������H2����
�ʴ�Ϊ��4P+5O2
2P2O5 ����������H2����
��2���������е���Ϣ������д��ѧ����ʽ�IJ��裻д��ע�ȣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+4H2
CH4+2H2O��
�ʴ�Ϊ��CO2+4H2
CH4+2H2O��
��3�����ݷ�ֹ����ЧӦ�Ĵ�ʩ��ֲ�����֣���ֲ���ݣ������̻��������������ú̿��ʯ�ͺ���Ȼ����ʹ�ã���������Դ����ѡBC��
| ||
B���ݣ����������⡱��ʵ����ۣ���֪Bʵ���и����õ�������Ϊ��������H2����
�ʴ�Ϊ��4P+5O2
| ||
��2���������е���Ϣ������д��ѧ����ʽ�IJ��裻д��ע�ȣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+4H2
| ||
| �� |
�ʴ�Ϊ��CO2+4H2
| ||
| �� |
��3�����ݷ�ֹ����ЧӦ�Ĵ�ʩ��ֲ�����֣���ֲ���ݣ������̻��������������ú̿��ʯ�ͺ���Ȼ����ʹ�ã���������Դ����ѡBC��
���������⿼��ѧ����д��ѧ����ʽ����������ǿ������ʶ������������

��ϰ��ϵ�д�
�����Ŀ
������ˮ��������̼������������Ҫ�Ļ�ѧ���ʡ�
��1�����������������������ԭ���� ��
��2��ˮ��һ�ֺܺõ��ܼ������м��ּ�������������ˮ���ܼ����ƶ��ɵģ��书�ܼ���Ч�ɷ����±���ʾ��
| �������� | ����� | ������Ư | Ư�� |
| ���� | ��Ч����۹�������ζ | Ưϴ���ʹɫ�ʸ����� | ����Ư���������� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
�� NaClO����Ԫ�صĻ��ϼ�Ϊ ��
�� ��ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������� ��
�� ������顱�롰Ư�������ܻ��á�����������ײ����ж���������ͬʱ���Ȼ��ƺ�ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������̼���������Ϊ�µ�ʱ������ȫ����̼�����ָ�����к����ٵ��������Ӷ�����̼�ر��Ƕ�����̼���ŷš��������ʽ���ϡ���̼��������е� ����������ţ���
�ٽ����������Ķ�����̼��ʯ��ˮ����
���ٿ�˽�ҳ��������������ͨ���߳���
�۹㷺ʹ��һ���Կ��ӡ�һ�������ϴ�
���õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô��漰��ӡ��