��Ŀ����
������ˮ�Ͷ�����̼������������Ҫ�����ʣ�
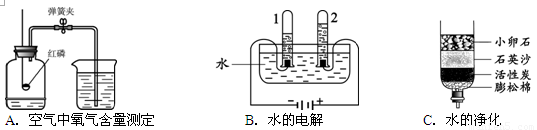
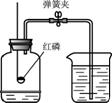
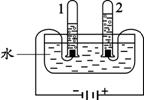
��1��A��B��C�����о�������ɺ����ʵ�ʵ�飮
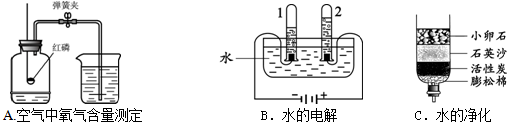
�ٹ���Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩣�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ��
��1��A��B��C�����о�������ɺ����ʵ�ʵ�飮

�ٹ���Aͼ��ʾʵ�飬����˵������ȷ����
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ����
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ����
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ��
��������1�����ݲⶨ����������������ʵ���ԭ����ע������������ڽ��и�ʵ��ʱ��װ�õ�������Ҫ�ã�ҩƷ����Ҫ�㣬������ȫ���ľ����ȵ�װ����ȴ�������ٴ��ɼж����ȵȣ�
��2���������������������̼�ķ�Ӧ�����ش�
��3�����ݺϳ�����[CO��NH2��2]�ķ�Ӧд��������Ӧ�ķ���ʽ��
��4�����ݵ�̼�ĺ�����з����жϣ�
��2���������������������̼�ķ�Ӧ�����ش�
��3�����ݺϳ�����[CO��NH2��2]�ķ�Ӧд��������Ӧ�ķ���ʽ��
��4�����ݵ�̼�ĺ�����з����жϣ�
����⣺��1��A��ʵ��ʱ����Ӧ���������ܽ������е��������ĵ�����A��ȷ��
B����ȼ����ǰ���õ��ɼмн��齺�ܣ���ֹ�������������ݳ�����B��ȷ��
C������Ϩ������̴��ɼУ�δ�ȵ�װ����ȴ�����£��ⶨ�����ƫС����C����
D�����ս���ƿ��ˮ�����ԼΪ�������������D��ȷ��
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ������������������еĶ�����̼��Ӧ�����˼�Ӳ��̼��ƺ�ˮ����Ӧ�ķ���ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��3���ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NH3
H2O+CO��NH2��2��
��4��A����������ʱ����ú������ú����ʹ�ã�����ú�������ĽӴ������ʹȼ�ճ�֣���Լ��Դ�������ŷţ���A��ȷ��
B����У��������͵��������շ��糧�����շ��磬������������ʡ��Դ����B��ȷ��
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ磬��ʡ��Դ����C��ȷ��
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ����D��ȷ��
�ʴ�Ϊ����1����C����H2�����ˣ���2��Ca��OH��2+CO2=CaCO3��+H2O����3��CO2+2NH3
H2O+CO��NH2��2����4��ABCD��
B����ȼ����ǰ���õ��ɼмн��齺�ܣ���ֹ�������������ݳ�����B��ȷ��
C������Ϩ������̴��ɼУ�δ�ȵ�װ����ȴ�����£��ⶨ�����ƫС����C����
D�����ս���ƿ��ˮ�����ԼΪ�������������D��ȷ��
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ������������������еĶ�����̼��Ӧ�����˼�Ӳ��̼��ƺ�ˮ����Ӧ�ķ���ʽ�ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��3���ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NH3
| ||
��4��A����������ʱ����ú������ú����ʹ�ã�����ú�������ĽӴ������ʹȼ�ճ�֣���Լ��Դ�������ŷţ���A��ȷ��
B����У��������͵��������շ��糧�����շ��磬������������ʡ��Դ����B��ȷ��
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ磬��ʡ��Դ����C��ȷ��
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ����D��ȷ��
�ʴ�Ϊ����1����C����H2�����ˣ���2��Ca��OH��2+CO2=CaCO3��+H2O����3��CO2+2NH3
| ||
������������Ҫ������������ˮ�Ͷ�����̼�ȳ������ʵ�֪ʶ�Լ�����ȵ㡰��̼���á��ĺ��壬�ѶȲ�����鱾�����յ�֪ʶ�ǽ��Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ