��Ŀ����
��ͭ���Ϻ�ɫ�����ֳ���ͭ����ͭ�ĺϽ��л�ͭ����ͭ�Ͱ�ͭ�ȣ�����Cu��Zn�ĺϽ�ƻ�ͭ����ͭ�������ĵ����Ժ���ʴ�ԣ��������������������Ϊ�˲ⶨij��ͭ��Ʒ��ͭ������������ȡ20�˸û�ͭ��Ʒ���뵽50��ϡ�����У�ǡ����ȫ��Ӧ����������0.2�ˣ���Ӧ�Ļ�ѧ����ʽΪ��Zn+H2S04�TZnS04+H2����������1���û�ͭ��Ʒ��ͭ������������
��2��ԭϡ������Һ����������������
���𰸡���������1����ͭ�е�ͭ�������ᷴӦ��п�����ᷴӦ�����ݷ�Ӧ�ķ���ʽ�������������Ϳ������п����������������û�ͭ��Ʒ��ͭ������������
��2������п�����ᷴӦ�ķ���ʽ���������������Ϳ������ϡ����������������������������������ļ��㹫ʽ���ԭϡ������Һ����������������
����⣺���ͭ��Ʒ�к�п������Ϊx��50��ϡ���������ʵ�����ΪY
Zn+H2SO4�TZnSO4+H2��
65 98 2
X Y 0.2g
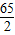 =
=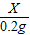
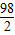 =
=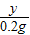
��ã�X=6.5g y=9.8g
��ͭ��Ʒ��ͭ��������20g-6.5g=13.5g
��ͭ��Ʒ��ͭ����������Ϊ��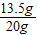 ×100%=67.5%
×100%=67.5%
ԭϡ������Һ��������������Ϊ�� ×100%=19.6%
×100%=19.6%
�𣺣�1����ͭ��Ʒ�к�ͭ67.5%����2��ԭ������Һ��������������Ϊ19.6%��
������������Ҫ�����˸��ݻ�ѧ����ʽ�ļ�����й��������������ļ��㣬�ѶȲ���ͨ����ϰ����Լ��ļ��㼼�ܣ�
��2������п�����ᷴӦ�ķ���ʽ���������������Ϳ������ϡ����������������������������������ļ��㹫ʽ���ԭϡ������Һ����������������
����⣺���ͭ��Ʒ�к�п������Ϊx��50��ϡ���������ʵ�����ΪY
Zn+H2SO4�TZnSO4+H2��
65 98 2
X Y 0.2g
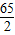 =
=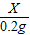
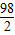 =
=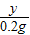
��ã�X=6.5g y=9.8g
��ͭ��Ʒ��ͭ��������20g-6.5g=13.5g
��ͭ��Ʒ��ͭ����������Ϊ��
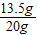 ×100%=67.5%
×100%=67.5%ԭϡ������Һ��������������Ϊ��
 ×100%=19.6%
×100%=19.6%�𣺣�1����ͭ��Ʒ�к�ͭ67.5%����2��ԭ������Һ��������������Ϊ19.6%��
������������Ҫ�����˸��ݻ�ѧ����ʽ�ļ�����й��������������ļ��㣬�ѶȲ���ͨ����ϰ����Լ��ļ��㼼�ܣ�

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
�����Ŀ