��Ŀ����
����Ŀ��ˮ�����౦�����Ȼ��Դ����Һ���ճ������ũҵ�����Ϳ����о��й㷺����;��
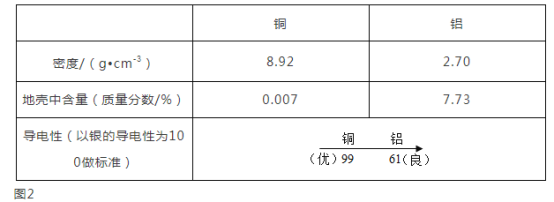
��1�������У�����ˮ������___________��������������
��2���ճ������У�Ϊ�˽���ˮ��Ӳ�ȣ�������________�ķ�����
��3��ˮ�����ճ�������������ܼ�����������Ͷ�뵽ˮ�У���ʹ��Һ�¶Ƚ��͵�����_______��
A NaOH���� B NH4NO3���� C ŨH2SO4 D CaO����
��4���ں�ۡ��ۺͷ���֮�佨����ϵ���ǻ�ѧѧ�Ƶ��ص㣬ij��Ӧ����ʾ��ͼ���£�
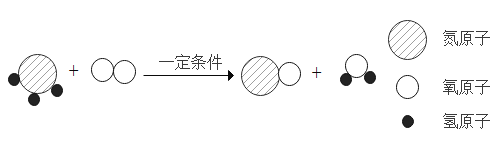
�ټ��������⡢������Ԫ�ص�������Ϊ��_____________
������������������������ǣ�______________���ѧʽ��
�۸÷�Ӧ�Ļ�ѧ����ʽΪ��_________________
���𰸡������ ��� B 3:14 NO��H2O 4NH3+5O2 4NO+6H2O
4NO+6H2O
��������
��1������ˮ�к���ˮ�����������ʵȶ������ʣ����ڻ����������
��2���ճ�������Ϊ�˽���ˮ��Ӳ�ȣ������õķ�������С������У�
��3��A��NaOH��������ˮ���ȣ���Һ�¶��������ߣ���ѡ�����
B��NH4NO3��������ˮ���ȣ���Һ�¶����Խ��ͣ���ѡ����ȷ��
C��ŨH2SO4����ˮ���ȣ���Һ�¶��������ߣ���ѡ�����
D��CaO������ˮ��Ӧ���ȣ���Һ�¶��������ߣ���ѡ�����
��ѡ��B��
��4���������ʵĻ�ѧʽΪNH3���⡢������Ԫ�ص�������Ϊ����1��3����14=3:14��
������������������������ǣ�һ��������ˮ��
���÷�Ӧ�ǰ�����������һ�������·�Ӧ����һ��������ˮ����Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2 4NO+6H2O��
4NO+6H2O��
���3:14��NO��H2O��4NH3+5O2 4NO+6H2O��
4NO+6H2O��

����Ŀ���ܶ����ֲ���ʯ������ɵ�ɽ���У�ʯ���ҵ���Ҫ�ɷ���̼��ƣ����������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O=Ca(HCO3)2��ij��ѧ��ȤС��ͬѧ��˼��������̼������Һ��ͨ��һ����������̼���Ƿ�������̼�����ƣ�NaHCO3�����������ǿ�ʼʵ��̽�����������Ƕ�����̼��̼����Ϊ���ʵ������
��������⣩
������Һ���������ʳɷ���ʲô��
���������ϣ�
��1��̼�����Σ���Ca(HCO3)2��Ba(HCO3)2��NaHCO3�ݶ�������ˮ
��2��Na2CO3��NaHCO3����Һ�ʼ��ԡ�BaCl2��Һ������
��������룩
����һ������ΪNa2CO3�������������Ϊ NaHCO3��������������Ϊ_____��
��ʵ����֤��
ʵ�鲽�� | ʵ������ | ʵ����� |
��1��ȡ��Ӧ����Һ�������Թ��У��μӼ���_____��Һ�� | ��Һ���ɫ | ����Һ�Լ��� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ�� | �а�ɫ�������� | ����_____������ |
��3��_____ | _____ | ���������� |
���ó����ۣ�������������֤��������̼ͨ��̼������Һ������̼�����ơ�
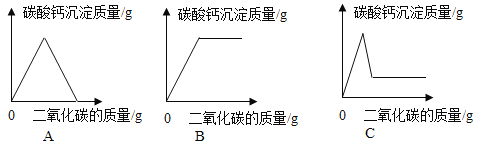
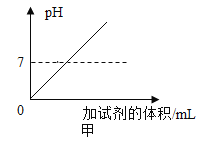
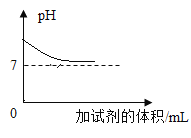
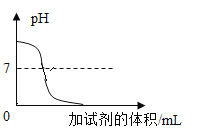
����չӦ�ã�ͬѧ�ǻ���ʵ�����ó���ʯ��ˮ���������̼���龰���������֪ʶ�������һ�����ij���ʯ��ˮ�в���ͨ�������̼���壬������һ��ͼ������ȷ��ӳ���ɵ�̼��Ƴ����������̼������֮��ı仯��ϵ_____������ĸ��ţ���