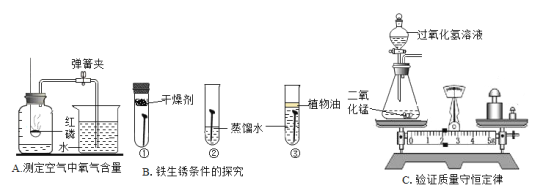
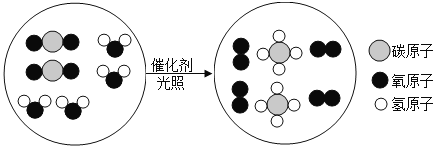
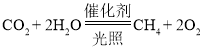��Ŀ����
����Ŀ�����ž��õķ�չ����Դ�ͻ�����Ϊ���������ע�����⡣
��1����ʯȼ����һ����Ҫ��Դ��������ú��ʯ�ͺ�___________�ȡ�
��2������һ�㲻ֱ����ʯ����ȼ�ϣ���������������ַе㲻ͬ��ͨ��________�ķ�ʽ�õ����ֲ�Ʒ��
��3���������м��������Ҵ���C2H5OH���������Ҵ����͡����Ҵ�������Ϊ����ȼ�ϣ���һ�ֽ��ܼ��ŵĴ�ʩ���Ҵ��ڿ�������ȫȼ�����ɶ�����̼��ˮ���仯ѧ����ʽΪ_________________��
���𰸡���Ȼ�� ���� 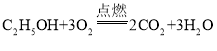
��������
��1��ú��ʯ�ͺ���Ȼ����������ʯȼ�ϣ������ڲ�����������Դ��
��2������ʯ�͵���ַе㲻ͬ����������������ֿ�����Ϊʯ�͵ķ������ܵõ����͡����͡�ú�͵ȣ�
��3���Ҵ��ڿ�������ȫȼ�����ɶ�����̼��ˮ���仯ѧ����ʽΪ��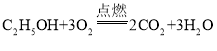 ��
��

����Ŀ��Ϊ̽��̼��ԭ����ͭ�����ʵ��������ȡľ̿�ۺ�����ͭ�ĸ�������1-2.5g����ͼ��ʾװ�ý���ʵ�顣
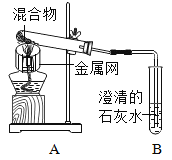
(1)ľ̿��ԭ����ͭ�Ļ�ѧ����ʽΪ__��ʵ�������Aװ�����Թ��ڲ�����������__��
(2)�ƾ��ƻ���ӽ������ֵ�Ŀ����____��
���������ϣ�������ͭ(CuO)Ϊ��ɫ���塣��̼��ԭ����ͭ�õ���ͭ�п��ܺ���������������ͭ��������ͭΪ��ɫ���壬����ϡ���ᷴӦ��Cu2O+H2SO4=CuSO4+H2O+Cu
���������飩ȡһ�����Ļ�������ͼ��ʾװ�ý���ʵ�顣
��� | ľ̿��������ͭ�������� | ��Ӧ�����ʵ���ɫ��״̬ | |
1 | 1��9 | ��ɫ�����н������� | ����������ɫ���� |
2 | 1��10 | ���к�������ɫ���� | |
3 | 1��11 | ���м�������ɫ���� | |
4 | 1��12 | ��ɫ���� | |
5 | 1��13 | ���н϶��ɫ���� | |
����������ͣ�(3)������ʵ����Եõ��Ľ�����___��������ѡ�
����˼�����ۣ�
(4)Ϊ�˼������ɵĺ�ɫ�������Ƿ���Cu2O�������Լ���____��
(5)��װ����̲��е�װ����������Ľ���ʵ�����ʱ�������õ��ɼмн���Ƥ�ܣ���Ϩ��ƾ��ƣ���������Ŀ�ij��˷�ֹʯ��ˮ�������ȵ��Թܣ�ʹ�Թ�ը���⣬�����Է�ֹ____��
����Ŀ���Ŵ���¯��ʯ��ZnCO3������ͭ��Cu2O����ľ̿�ۻ�Ϻ���ȵ�һ���¶ȣ����Եõ�һ������ƽ��ӵ�п��ͭ�ĺϽ𣬲�������������ð��ƽ𣮻�ѧ��ȤС����ʵ���ҽ�����ľ̿�ۻ�ԭ������ͭ��ʵ�飨��Ҫ��Ӧ��C+Cu2O![]() 2Cu+CO�������۲쵽��Ӧ��Ĺ��岻��ȫ���Ϻ�ɫ�����ǶԸù���ijɷֽ�����ʵ��̽����
2Cu+CO�������۲쵽��Ӧ��Ĺ��岻��ȫ���Ϻ�ɫ�����ǶԸù���ijɷֽ�����ʵ��̽����
��������⣩�ù���ijɷ���ʲô��
���������ϣ���Cu���Ϻ�ɫ���� ��Cu2O�Ǻ�ɫ���� ��Cu2O+H2SO4��ϡ��=CuSO4+Cu+H2O
����������裩
����һ��Cu �������Cu��Cu2O ��������Cu��C �����ģ�_______________
��ȤС����Ϊ����һ����ȷ����Ϊ________________________��
��ʵ��̽����
ʵ����� | ʵ������ | ʵ����� |
����һ��ȡ�������������ձ��У���������ϡ���ᣬ�۲���Һ��ɫ | ��Һ��_____ɫ | ����������ȷ |
��������Ѳ���һ�����û������ˣ������������������ֽ�ϣ��۲������ɫ | ����Ϊ�Ϻ�ɫ����ɫ | ����____��ȷ |
��ʵ�鷴˼�������ܷ�Ӧ�������ȡ���Ӧ��Ӵ�����ͷ�Ӧ�¶ȵ����ص�Ӱ�죬��������֮��ķ�Ӧ��������ȫ���У�