��Ŀ����
 ˮ������֮Դ������ˮ��Դ��ÿ������Ӧ�������Σ���ش������й����⣺
ˮ������֮Դ������ˮ��Դ��ÿ������Ӧ�������Σ���ش������й����⣺��1��Ӳˮ����������������ܶ��鷳�������п���
��2��������һЩ��ˮ�ķ��������п�ʹӲˮ�����ˮ���ǣ�
�ٹ��� ������ �۳��� �����
��3���ҹ��ǵ�ˮ��Դ�dz����Ĺ��ң�����ˮ��Դ��ÿ����������κ�����������Ϊ�����˷�ˮ����
A��ϴ�ֲ�����ʱ������ˮ��ͷ B����ϴ��ˮ����
C����ϴ��ˮ����� D��������ˮ����Ϊ���ϳ�ˮ����
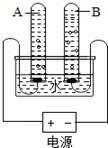
��4������ͼ��ʾװ�ý���ʵ�飬һ��ʱ���A��B�Թ��е����������Ϊ
��������1����������Ӳˮ����ˮ���õķ������÷���ˮ���н��
��2����������Ӳˮ�ķ������н��
��3�����ݳ������˷�ˮ���������н��
��4�����ݵ��ˮ��֪ʶ���з��������ˮͨ�����ֱ���磬�����������������������������������2��1�������������������������������н��
��2����������Ӳˮ�ķ������н��
��3�����ݳ������˷�ˮ���������н��
��4�����ݵ��ˮ��֪ʶ���з��������ˮͨ�����ֱ���磬�����������������������������������2��1�������������������������������н��
����⣺��1������Ӳˮ����ˮ���õķ������÷���ˮ��������ĭ�϶������ˮ�����ٵ�Ӳˮ���������ˮ��
��2�������ܳ�ȥˮ�����������ʣ������dz�ȥˮ�нϴ���������ʣ����ܽ�Ӳˮ���������������п���ʹӲˮ�����ˮ������ڢܣ�
��3��A��ϴ�ֲ�����ʱ������ˮ��ͷ���˷�ˮ��Դ��B����ϴ��ˮ��������һˮ���ã����Խ�Լ��ˮ��
C����ϴ��ˮ���������һˮ���ã����Խ�Լ��ˮ��D��������ˮ����Ϊ���ϳ�ˮ���»��˷�ˮ��Դ��
��ѡ��AD��
��4�����ˮͨ�����ֱ���磬�����������������������������������������������2��1������A�Թ����Դ������������A�Թܲ���������B�Թ����Դ�ĸ���������B�Թܲ����������������п�ȼ�ԣ�ȼ�շ�������ɫ�Ļ��棬���Կ���ȼ�ŵ�ľ�����飬��Ӧ�Ļ�ѧ����ʽΪ2H2+O2
2H2O����ʵ��֤����ˮ�����⡢��Ԫ����ɵģ� ��ʵ������з�����Ӧ�Ļ�ѧ����ʽΪ2H2O
2H2��+O2�������1��2��������ȼ�ŵ�ľ����2H2+O2
2H2O���⡢��Ԫ�أ�2H2O
2H2��+O2����
��2�������ܳ�ȥˮ�����������ʣ������dz�ȥˮ�нϴ���������ʣ����ܽ�Ӳˮ���������������п���ʹӲˮ�����ˮ������ڢܣ�
��3��A��ϴ�ֲ�����ʱ������ˮ��ͷ���˷�ˮ��Դ��B����ϴ��ˮ��������һˮ���ã����Խ�Լ��ˮ��
C����ϴ��ˮ���������һˮ���ã����Խ�Լ��ˮ��D��������ˮ����Ϊ���ϳ�ˮ���»��˷�ˮ��Դ��
��ѡ��AD��
��4�����ˮͨ�����ֱ���磬�����������������������������������������������2��1������A�Թ����Դ������������A�Թܲ���������B�Թ����Դ�ĸ���������B�Թܲ����������������п�ȼ�ԣ�ȼ�շ�������ɫ�Ļ��棬���Կ���ȼ�ŵ�ľ�����飬��Ӧ�Ļ�ѧ����ʽΪ2H2+O2
| ||
| ||
| ||
| ||
�����������ѶȲ����˽⾻��ˮ�ij��÷�����Ӳˮ����ˮ�ļ�����ת������������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 ˮ������֮Դ��û��ˮ��û��������
ˮ������֮Դ��û��ˮ��û��������
